
| Ń”Ļī | øıäĢõ¼ž | Ę½ŗāŅĘ¶Æ·½Ļņ | µ¼µēÄÜĮ¦ |
| A | ¼ÓČėÕōĮóĖ® | ÄęĻņŅĘ¶Æ | ¼õČõ |
| B | ¼ÓČėÉŁĮæ±ł“×Ėį | ÕżĻņŅĘ¶Æ | ŌöĒæ |
| C | ¼ÓČėÉŁĮæCH3COONa¹ĢĢå | ÕżĻņŅĘ¶Æ | ŌöĒæ |
| D | ¼ÓČėÉŁĮæ0.1mol•L-1µÄĻ”ĮņĖį | ÄęĻņŅĘ¶Æ | ¼õČõ |
| A£® | A | B£® | B | C£® | C | D£® | D |
·ÖĪö ČÜŅŗÖŠĄė×ÓÅضČŌ½“ó£¬ČÜŅŗµÄµ¼µēŠŌŌ½Ē棬½įŗĻÓ°ĻģµēĄėĘ½ŗāµÄŅņĖŲ½ā“š£®
½ā“š ½ā£ŗA£®¼ÓĖ®“Ł½ųČõµē½āÖŹµÄµēĄė£¬ŌņµēĄėĘ½ŗāÕżĻņŅĘ¶Æ£¬µ«ŹĒČÜŅŗÖŠĄė×ÓÅØ¶Č¼õŠ”£¬µ¼µēŠŌ¼õČõ£¬¹ŹA“ķĪó£»
B£®¼ÓČėÉŁĮæ±ł“×Ėį£¬CH3COOHµÄÅضČŌö“ó£¬Ź¹µēĄėĘ½ŗāÕżĻņŅĘ¶Æ£¬ČÜŅŗÖŠĄė×ÓÅضČŌö“󣬵¼µēŠŌŌöĒ棬¹ŹBÕżČ·£»
C£®¼ÓČėÉŁĮæCH3COONa¹ĢĢ壬ÓɵēĄėĘ½ŗāæÉÖŖ£¬c£ØCH3COO-£©Ōö“ó£¬ŌņµēĄėĘ½ŗāÄęĻņŅĘ¶Æ£¬ČÜŅŗÖŠĄė×ÓÅضČŌö“󣬵¼µēŠŌŌöĒ棬¹ŹC“ķĪó£»
D£®¼ÓČėÉŁĮæ0.1mol•L-1Ļ”ĮņĖį£¬c£ØH+£©Ōö“󣬵ēĄėĘ½ŗāÄęĻņŅĘ¶Æ£¬ČÜŅŗÖŠĄė×ÓÅضČŌö“󣬵¼µēŠŌŌöĒ棬¹ŹD“ķĪó£»
¹ŹŃ”B£®
µćĘĄ ±¾Ģāæ¼²éµēĄėĘ½ŗāµÄŅĘ¶Æ”¢ČÜŅŗµÄµ¼µēŠŌ£¬Ć÷Č·Ó°ĻģĘ½ŗāŅĘ¶ÆµÄŅņĖŲ¼“æɽā“š£¬×¢ŅāĄūÓĆ¼ÓĖ®“Ł½ųµēĄė¼°Ķ¬Ąė×ÓŠ§Ó¦Ą“·ÖĪö½ā“š£¬ĢāÄæÄŃ¶Č²»“ó£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
 ·Ē½šŹōµ„ÖŹAŗĶ½šŹōµ„ÖŹMæÉ·¢ÉśČēĶ¼ĖłŹ¾µÄ×Ŗ»Æ¹ŲĻµ£¬LµÄŃęÉ«ŹĒ»ĘÉ«£¬ĶłLµÄČÜŅŗÖŠ¼ÓČėĻ”ŃĪĖįŗĶBaCl2ČÜŅŗ»įÉś³É°×É«³Įµķ£®
·Ē½šŹōµ„ÖŹAŗĶ½šŹōµ„ÖŹMæÉ·¢ÉśČēĶ¼ĖłŹ¾µÄ×Ŗ»Æ¹ŲĻµ£¬LµÄŃęÉ«ŹĒ»ĘÉ«£¬ĶłLµÄČÜŅŗÖŠ¼ÓČėĻ”ŃĪĖįŗĶBaCl2ČÜŅŗ»įÉś³É°×É«³Įµķ£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Cu £ØCuO£©£¬¼ÓŃĪĖį£¬¹żĀĖ | B£® | Na2CO3 £ØNaHCO3£©¹ĢĢ壬¼ÓČČ | ||
| C£® | KNO3 £ØK2SO4£©£¬¼ÓBaCl2ČÜŅŗ£¬¹żĀĖ | D£® | MgO £ØAl2O3£©£¬¼ÓÉÕ¼īČÜŅŗ£¬¹żĀĖ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ĻņĀČ»ÆŃĒĢśČÜŅŗÖŠ¼ÓČėĒāŃõ»ÆÄĘČÜŅŗ£¬ĻČÓŠ°×³öĻÖ£¬ŗó°×É«³ĮµķÖš½„ĻūŹ§ | |
| B£® | ½«Ķʬ·ÅČĖĀČ»ÆĢśČÜŅŗ£¬øÖʬ±ķĆęÉś³ÉŅų°×É«µÄĢś | |
| C£® | ŌŚĀČ»ÆĢśČÜŅŗÖŠµĪČėĒāŃõ»ÆÄĘČÜŅŗ£¬ÓŠŗģŗÖÉ«³Įµķ³öĻÖ | |
| D£® | ½«Ķ·Ū¼ÓČĖĄäµÄÅØĻõĖįÖŠ»į·¢Éś¶Ū»Æ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ŗ¬ÓŠ6.02”Į1023øöŗ¤Ō×ÓµÄŗ¤ĘųŌŚ±ź×¼×“æöĻĀµÄĢå»żŌ¼ĪŖ11.2 L | |
| B£® | ŌŚ³£ĪĀ³£Ń¹ĻĀ£¬11.2 L Cl2ŗ¬ÓŠµÄ·Ö×ÓŹżĪŖ0.5”Į6.02”Į1023 | |
| C£® | 25”ę£¬1.01”Į105Pa£¬64 g SO2ÖŠŗ¬ÓŠµÄŌ×ÓŹżĪŖ3”Į6.02”Į1023 | |
| D£® | ±ź×¼×“æöĻĀ£¬11.2 L H2Oŗ¬ÓŠµÄ·Ö×ÓŹżĪŖ0.5”Į6.02”Į1023 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
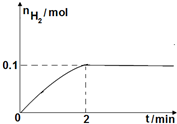 ĮņŅ»µāŃ»··Ö½āĖ®ÖĘĒāÖ÷ŅŖÉę¼°ĻĀĮŠ·“Ó¦£ŗ
ĮņŅ»µāŃ»··Ö½āĖ®ÖĘĒāÖ÷ŅŖÉę¼°ĻĀĮŠ·“Ó¦£ŗ²éæ““š°øŗĶ½āĪö>>
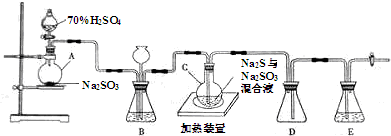
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
| µŚŅ»·Ż | µŚ¶ž·Ż | µŚČż·Ż | |
| ѳʷµÄÖŹĮæ/g | 12.60 | 18.90 | 28.00 |
| ¶žŃõ»ÆĮņµÄĢå»ż/L | 1.12 | 1.68 | 2.24 |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com