
����Ŀ����1�������о����У���NaOH����Na2S����(NH4)2S����Na2O2����C2H2����SiC�����мȺ������Ӽ��ֺ��зǼ��Թ��ۼ������Ӿ�����__________�����мȺ������Ӽ����ֺ��м��Թ��ۼ�����λ�������Ӿ�����___________�����к��м��Թ��ۼ��ͷǼ��Թ��ۼ��ķǼ��Է�����____________����������ԭ�Ӿ������____________��
��2��Feԭ�ӻ�������Χ�н϶���������Ŀչ��������һЩ���ӻ������γ�����
�� ��Feԭ�ӻ������γ������ķ��ӻ����Ӷ��߱��Ľṹ�ص���_______________��
�� ������������ӡ�Fe(CN)6��4-�е�����CN-��Cԭ�ӵ��ӻ����������________��д��һ����CN��Ϊ�ȵ�����ĵ��ʷ��ӵĽṹʽ_______��
��3�����Ȼ���������Ϊ���壬�۵�282�棬�е�315�棬��300��������������������ˮ��Ҳ���������ѣ���ͪ���л��ܼ����ݴ��ж����Ȼ�����������Ϊ_________________��
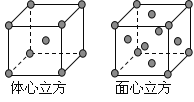
��4���������ľ����ڲ�ͬ�¶��������ֶѻ���ʽ�������ֱ�����ͼ��ʾ������������������������������ʵ�ʺ��е�Feԭ�Ӹ���֮��Ϊ_______________________��
���𰸡� �� �� �� �� ���й¶Ե��� sp N��N ���Ӿ��� 1:2
����������������1�����Ӽ�ͨ�����Ӽ��γɵľ��������Ӿ��壬���Ӽ�ͨ�����Ӽ��������γɵľ����Ƿ��Ӿ��壬ԭ�Ӽ�ͨ�����ۼ��γɵĿռ���״�ṹ�ľ�����ԭ�Ӿ��壬������Ӽ����ۼ����γɷ������
��2���ٺ��пչ���ͺ��йµ��ӶԵ�ԭ��֮�����γ���λ����
��CN����Cԭ�Ӽ۲���ӶԸ�����2���Һ���һ���µ��Ӷԣ����ݼ۲���ӶԻ��������ж�Cԭ���ӻ����ͣ�ԭ�Ӹ�����ȡ��۵�������ȵ�����Ϊ�ȵ����壻
��3�����Ӿ����۷е�ϵͣ�
��4�����þ�̯�ּ���ÿ��������ԭ�Ӹ�����
��⣺��1����NaOH�к������Ӽ��ͼ��Լ�����Na2S��ֻ�����Ӽ�����(NH4)2S�к������Ӽ��ͼ��Լ�����Na2O2�к������Ӽ��ͷǼ��Լ�����C2H2�к��м��Լ��ͷǼ��Լ�����SiC��ֻ�м��Լ��������мȺ������Ӽ��ֺ��зǼ��Թ��ۼ������Ӿ����ǹ������ƣ����мȺ������Ӽ����ֺ��м��Թ��ۼ�����λ�������Ӿ�����(NH4)2S����Ȳ��ֱ���νṹ��������к��м��Թ��ۼ��ͷǼ��Թ��ۼ��ķǼ��Է�������Ȳ����������ԭ�Ӿ������SiC��
��2���ٺ��пչ���ͺ��йµ��ӶԵ�ԭ��֮�����γ���λ����Feԭ�Ӻ��пչ������Feԭ���ܺͺ��йµ��ӶԵ�ԭ�����γ���λ����
��CN-��Cԭ�Ӽ۲���ӶԸ�����2���Һ���һ���µ��Ӷԣ����ݼ۲���ӶԻ��������ж�Cԭ���ӻ�����Ϊsp��ԭ�Ӹ�����ȡ��۵������ֱ���ȵ�����Ϊ�ȵ����壬����������к���2��ԭ�ӡ��۵�������10���뵪�����ӻ�Ϊ�ȵ����壬�������Ӻ�����������ṹʽΪN��N��
��3�����Ӿ����۷е�ϵͣ����������Ϣ֪���Ȼ����۷е�ϵͣ�Ӧ���Ƿ��Ӿ�����
��4��������������������Feԭ�Ӹ���=8��1/8+6��1/2=4����������������Feԭ�Ӹ���=1+8��1/8=2��������������������Feԭ�Ӹ�������������������Feԭ�Ӹ���֮��=2��4=1��2��


 ����ѧ����ϵ�д�
����ѧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Ҫ����գ�
(1)��֪R2�����ӵĺ�����n�����ӣ�R��������ΪM����mg R2�������ﺬ�е��ӵ����ʵ���Ϊ ____________mol��
(2)XԪ������������Ӧ��ˮ����ΪH3XO4��������Ӧ����̬�⻯��Ϊ_____ ��
(3)��֪ͬ����X��Y��Z����Ԫ�ص�����������Ӧˮ����������ǿ������˳��ΪHXO4��H2YO4��H3ZO4����Ԫ�طǽ�������ǿ����˳��Ϊ��____________ ����̬�⻯����ȶ�����ǿ����˳��Ϊ��______________ ��
(4).�ס������ַ�Ԫ�أ��ټױ���������H2���ϣ��ڼ�ԭ�������ҵ������ӷ����û���Ӧ���ۼ�����������Ӧ��ˮ�������Ա��ҵ�����������Ӧ��ˮ��������ǿ������ij������Ӧʱ����ԭ�ӵõ�����Ŀ���ҵĶࣻ�ݼĵ����ۡ��е���ҵĵ͡�
��˵���ķǽ����Ա��ҵķǽ�����ǿ����________________
(5)�±���ij��ȤС��ͨ��ʵ���õ���ͬ�������ϡ����������Ӧ��ʵ�����ݣ�
ʵ����� | ��������/g | ����״̬ | c(H2SO4) mol/L | ʵ���¶�/�� | ������ʧ��ʱ��/s |
1 | 0.10 | ˿ | 0.7 | 25 | 240 |
2 | 0.10 | ˿ | 1.0 | 25 | 190 |
3 | 0.10 | ��ĩ | 1.0 | 25 | 120 |
4 | 0.10 | ��ĩ | 1.0 | 40 | 40 |
�����������ݣ��ش��������⣺
��ʵ��1��
��ʵ��2��3�ɵó��Ľ�����_________________________��
��ʵ��3��4�ɵó��Ľ�����______________________��
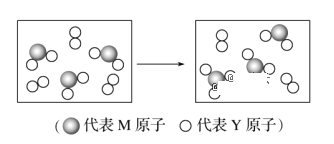
(6)��ͼ��ʾ��M��Y��Ԫ����ɵ��������������һ�������µ��ܱ������г�ַ�Ӧǰ���ת����ϵ����д����ת�����̵Ļ�ѧ����ʽ��______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������������ҡ������ˮ�����DZ����ˮ��Դ�������̲��ŷḻ�Ļ�ѧ��Դ��
I.��1����ˮ�����ô�����ͨ����Ca2+��Mg2+��SO42-�����ʣ�Ϊ�˵õ����Σ������Լ�������Ⱥ�˳����ȷ����________
A��BaCl2��Na2CO3��NaOH��HCl B��NaOH��BaCl2��Na2CO3��HCl
C��BaCl2��NaOH��Na2CO3��HCl D�� Na2CO3��NaOH��BaCl2��HCl
��2��Ϊ�˼��龫�����Ƿ���SO42-����ȷ�ķ�����____________________________��
II.��������ȡ���������ͼ��ʾ
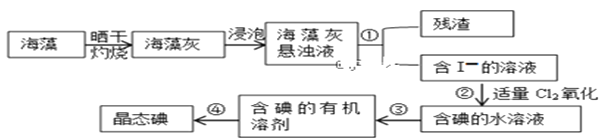
��3�����չ����У���ʹ�õ��ģ����������⣩ʵ��������____________
A���Թ� B�������� C������ǯ D�������� E���ƾ��� F�����ż�
��4��ָ����ȡ��Ĺ������й�ʵ��������ƣ���________��__________��_________
��5������ڷ�Ӧ�����ӷ���ʽΪ___________________���ù���������Ҳ������H2O2���������ʵ�����I-ת��ΪI2������Cl2��H2O2�����ʵ���֮��Ϊ__________
��6�����й��ں�����ȡ���˵������ȷ����_________
A��������л��ܼ������Ϻ�ɫ
B�����������ȷų��²�Һ�壬Ȼ���ٴ��¿ڷų��ϲ�Һ��
C��������ʱ���¶ȼƵ�ˮ����Ӧ����Һ�����µ����ܴ�����������ƿ�ĵײ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������һ������Ч��Դ���Լ״�Ϊԭ�Ͽ�����ȡ�������ش�����������
��1���״�ˮ������������
����Ӧ����CH3OH(g)![]() CO(g)+2H2(g) ��H =+90.7kJ/mol
CO(g)+2H2(g) ��H =+90.7kJ/mol
��CO(g)+H2O(g)![]() CO2(g)+H2(g) ��H =-41.2 kJ/mol
CO2(g)+H2(g) ��H =-41.2 kJ/mol
����Ӧ����CO(g)+3H2(g)![]() CH4(g)+H2O(g) ��H =+206.3 kJ/mol
CH4(g)+H2O(g) ��H =+206.3 kJ/mol
��״�ˮ�����������ⷴӦ(��Ӧ��)��CH3OH(g) +H2O(g)![]() CO2(g)+3H2(g)�� ��H=_________����Ӧ����lmolCO��lmolˮ������Ӧ�ĵĻ��ΪE1kJ����÷�Ӧ������Ļ��Ϊ_________kJ��
CO2(g)+3H2(g)�� ��H=_________����Ӧ����lmolCO��lmolˮ������Ӧ�ĵĻ��ΪE1kJ����÷�Ӧ������Ļ��Ϊ_________kJ��
��2��ʵ����ģ��״�ˮ��������������̣��ϳ������n(CH3OH)��n(H2O) =1��1ʱ����ϵ�м״���ƽ��ת�������¶Ⱥ�ѹǿ�Ĺ�ϵ��ͼ��ʾ��

���÷�Ӧ��ƽ�ⳣ������ʽΪ_______________��
�����¶�Ϊ250����ѹǿΪP2ʱ����Ӧ��ƽ��ʱH2���������Ϊ______________��
��ͼ�е�ѹǿ��С�����˳����________________��
������������Ӧ�����������IJ�����ѹǿ�������___________����ԭ����__________________��
��3��MFC30����ȼ�ϵ������̼����Ϊ�����(�ṩCO32-)�ĸ�����ȼ�ϵ�أ������ĵ缫��ӦʽΪ_____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����Ʒ���ʢ�п������ܱ������У�����Na2O����Na2O2������������������������(����)
A.��Ӧ�¶�B.��������C.�����Ĵ�СD.�Ƶ�״̬(s��l)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������NaNO2��һ�ֹ�ҵ�Σ�������ʳ�������ж���ij����С������ø�����صζ����ⶨij��Ʒ��NaNO2��������������ȡ1.0g����Ʒ����ƿ�У���������ˮ�ܽ⣬Ȼ����0.1000mol/L��KMnO4��Һ(����ϡH2SO4�ữ)���вⶨ�����ظ���������2-3�����ش��������⣺
��1���ζ������з�Ӧ�����ӷ���ʽΪ_____________��
��2���ζ��յ���жϷ�����______________��
��3�����ζ��յ�ʱƽ������20.00mL����Һ������Ʒ��NaNO2����������Ϊ___________��
��4�����в����п���ʹ�ⶨ���ƫ�ߵ���______________��
A.�ζ������ζ��ܼ��촦��һ����Һ��
B.�ζ��ܼ��첿�ֵζ�ǰ�����ݣ��ζ�����������
C.�ζ�ǰ���ӵζ��̶ܿ������ζ��յ�ƽ�ӿ̶���
D.��ƿ������ˮϴ�Ӻ�ֱ�Ӽ������Һ���еζ�
��5����С�黹�����ʵ��֤��������������NaNO2���������ԣ�ʵ�����Ϊ___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ˮ��Դ�����þ��зdz�������ǰ�����Ӻ�ˮ�п���ȡ���ֻ���ԭ�ϡ�
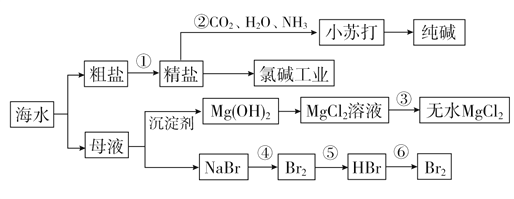
(1)��ˮ���������ķ�����__________________________(д�����ּ���)��
(2)�����к���Ca2����Mg2����SO![]() �����ʣ�����ʱ�����Լ�Ϊ��
�����ʣ�����ʱ�����Լ�Ϊ��
A������ B��BaCl2��Һ C��NaOH��Һ D��Na2CO3��Һ
�����Լ���˳����__________________��
(3)������У�������Һ��Ӧ��ͨ��________����ͨ��________��
(4)�ȼҵ�У����Դ���������ĵ缫������ҺpHֵ________(������С�����䡱)���ò�����պŨ��ˮ�����������������壬���ֲ����������̣����̵���Ҫ�ɷ���________________________��
(5)ʵ�������У���ѡ��________��Ϊ�����������Ȼ�þ��Һ�еõ���ˮ����IJ���Ϊ_____________________________________��
(6)�������SO2ˮ��Һ�����嵥�ʣ������ʿɴ�93%����Ӧ�����ӷ���ʽΪ______________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ѡ�ý�������ʱ�����·�����Ҫ���ǵ���(����)
����Ҫ��;�����������ʡ���ѧ���ʡ����۸����ӹ��Ѷȡ����ճ�ά�������Ի�����Ӱ��
A.�٢ڢ�B.�ۢܢ�C.�٢ݢ�D.�٢ڢۢܢݢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��(1)����(![]() )��С�մ�(
)��С�մ�(![]() )�㷺Ӧ����ʳƷ�ӹ����մ����ԭ���к���С�մ��ں決���ȹ��̣�С�մ����ֽⷴӦ���÷�Ӧ�Ļ�ѧ����ʽ��______���մ���ɿ����ʵ���������θ��(��Ҫ�ɷ�����)����֢״���÷�Ӧ�Ļ�ѧ����ʽ��_______��
)�㷺Ӧ����ʳƷ�ӹ����մ����ԭ���к���С�մ��ں決���ȹ��̣�С�մ����ֽⷴӦ���÷�Ӧ�Ļ�ѧ����ʽ��______���մ���ɿ����ʵ���������θ��(��Ҫ�ɷ�����)����֢״���÷�Ӧ�Ļ�ѧ����ʽ��_______��

(2)ijͬѧ������ͼװ��(�г���������ȥ)�о������С�մ�����ʡ�
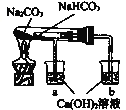
�ٸ�ʵ���Ŀ����_______��
�ڹ۲쵽�а�ɫ�������ɵ��ձ���_________(����ĸ���)��
��ʵ�������__________��
��Ϊ��һ�������о�̼�����Ƶ����ʣ���ͬѧ����ƽȷ����![]() ̼�����ƣ���������Ϊ
̼�����ƣ���������Ϊ![]() �������м��ȣ���ȴ������������������ʣ������������Ϊ______
�������м��ȣ���ȴ������������������ʣ������������Ϊ______![]() ����ʵ�ʳƵõ������ȸ���ֵҪ���ܵ�ԭ����________���Ľ��IJ���������_______________��
����ʵ�ʳƵõ������ȸ���ֵҪ���ܵ�ԭ����________���Ľ��IJ���������_______________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com