
�������繤ҵ���õķ�չ���˿ڵľ�����ȫ����Դ���ż�������������Խ��Խ���ص����⣬��ν��ʹ�����CO2�ĺ�������Ч�ؿ�������CO2������ȫ������ձ����ӡ�
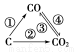
��1����ͼΪC����������ı仯��ϵͼ�������仯���û���Ӧ�����仯ѧ����ʽ��Ϊ______________________________________��
ͼ�б仯������Щ�����ȷ�Ӧ________������ţ���
��2���״���һ�ֿ�������Դ�����п�����Ӧ�õĹ���ǰ������ҵ�Ͽ������·����ϳɼ״���
����һ��CO��g����2H2��g��??CH3OH��g��
��������CO2��g����3H2��g��??CH3OH��g����H2O��g��
��25����101 kPa�£�1���״���ȫȼ�շ���22.68 kJ��д���״�ȼ�յ��Ȼ�ѧ����ʽ��_____________________________________________��
ij�������糧CO2������ŷ�����2 200��֣�������CO2��ȫת��Ϊ�״������������ɴ˻�õļ״���ȫȼ�շ���Լ��________kJ��������λ��Ч���֣���
��3��������ұ������������һ����Ӧ�ǽ�ԭ�Ͻ��ʯת����TiO2�����ʯ����2C��2Cl2����,TiCl4��2CO����֪��C��s����O2��g��=CO2��g������H����393.5 kJ��mol��1
2CO��g����O2��g��=2CO2��g������H����566 kJ��mol��1
TiO2��s����2Cl2��g��=TiCl4��s����O2��g������H����141 kJ��mol��1
��TiO2��s����2Cl2��g����2C��s��=TiCl4��s����2CO��g������H��________��
��4�����������ھ�������������ˮ������������ҵ�������ΪƯ�����������������������ҿ������������е��ʷ�Ӧ���磺
6Ag��s����O3��g��=3Ag2O��s������H����235.8 kJ��mol��1��
��֪��2Ag2O��s��=4Ag��s����O2��g����H����62.2 kJ��mol��1��
��O3ת��ΪO2���Ȼ�ѧ����ʽΪ_________________________��
��1��C��H2O��g������,H2��CO���������ɣ�����CuO��FeO��SiO2�ȷ�Ӧ�����٢�
��2��CH3OH��l����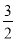 O2��g��=CO2��g����2H2O��l������H����725.76 kJ��mol��1��3.63��1014
O2��g��=CO2��g����2H2O��l������H����725.76 kJ��mol��1��3.63��1014
��3����80 kJ��mol��1
��4��2O3��g��=3O2��g������H����285 kJ��mol��1
����������1������Ϣ��֪��Ӧ�����û���Ӧ������ӦΪ���ȷ�Ӧ����2��1 mol�״�ȼ�շų�����22.68 kJ��32��725.76 kJ��������Ϣ������ˮΪҺ̬������̼ԭ���غ�n��CH3OH����n��CO2����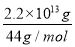 ��5��1011 mol���ų�����725.76 kJ��mol��1��5��1011 mol��3.63��1014 kJ����3������֪�Ȼ�ѧ����ʽ��Ŵ�ǰ����ֱ�Ϊ�٢ڢ���������������2���ɵ�����ʽ��������H����141 kJ��mol��1������566 kJ��mol��1��������393.5 kJ��mol��1����2����4������д������Ӧ�Ļ�ѧ����ʽ2O3��g��=3O2��g����ǰʽ��2����ʽ��3���ɵ������Ȼ�ѧ����ʽ��
��5��1011 mol���ų�����725.76 kJ��mol��1��5��1011 mol��3.63��1014 kJ����3������֪�Ȼ�ѧ����ʽ��Ŵ�ǰ����ֱ�Ϊ�٢ڢ���������������2���ɵ�����ʽ��������H����141 kJ��mol��1������566 kJ��mol��1��������393.5 kJ��mol��1����2����4������д������Ӧ�Ļ�ѧ����ʽ2O3��g��=3O2��g����ǰʽ��2����ʽ��3���ɵ������Ȼ�ѧ����ʽ��


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2014��߿���ѧ���ָ�ϰָ��Ԥ��Ѻ����ϰ�������� �������棩 ���ͣ�ѡ����
��������ȡ����Ӧ���ǣ�������
��CH3CH=CH2��Br2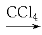 CH3CHBrCH2Br
CH3CHBrCH2Br
��CH3CH2OH��3O2 2CO2��3H2O
2CO2��3H2O
��CH3COOH��CH3CH2OH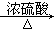 CH3COOCH2CH3��H2O
CH3COOCH2CH3��H2O
��C6H6��HNO3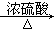 C6H5NO2��H2O
C6H5NO2��H2O
A���٢� B���ۢ� C���٢� D���ڢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014��߿���ѧ���ָ�ϰר�������3 Ԫ�ؼ��仯������ϰ���������棩 ���ͣ�ʵ����
ijѧϰС���������ͼװ����֤��������Ļ�ѧ���ʡ�
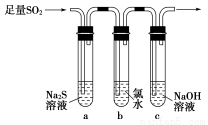
��1����˵������������������Ե�ʵ������Ϊ_________________________��
��2��Ϊ��֤��������Ļ�ԭ�ԣ���ַ�Ӧ��ȡ�Թ�b�е���Һ�ֳ����ݣ��ֱ��������ʵ�顣
�����������һ����Һ�м���AgNO3��Һ���а�ɫ��������
����������ڶ�����Һ�м���Ʒ����Һ����ɫ��ȥ
�����������������Һ�м���BaCl2��Һ��������ɫ����
���������к�������________�������������������������������Թ�b�з�����Ӧ�����ӷ���ʽΪ____________________________________________��
��3����ͨ������������Թ�c����Һ������ʱ������Һ��c��Na������________���ú�����Ũ�ȵĴ���ʽ��ʾ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014��߿���ѧ���ָ�ϰר�������2 ��ѧ����������ϰ���������棩 ���ͣ������
��Դ�Ŀ����������������Ŀɳ�����չϢϢ��ء�
��.��֪��Fe2O3��s����3C��s��=2Fe��s����3CO��g��
��H1��a kJ��mol��1
CO��g����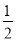 O2��g��=CO2��g������H2��b kJ��mol��1
O2��g��=CO2��g������H2��b kJ��mol��1
4Fe��s����3O2��g��=2Fe2O3��s������H3��c kJ��mol��1
��C��ȼ������H��________kJ��mol��1��
��.��1������ԭ��صĹ���ԭ�������л�ѧ��Ӧ�������Ͽ�����Ƴ�ԭ��ص���________������ţ���
A��C��s����CO2��g��=2CO��g��
B��NaOH��aq����HCl��aq��=NaCl��aq����H2O��l��
C��2H2O��l��=2H2��g����O2��g��
D��2CO��g����O2��g��=2CO2��g��
�������ڵ�K2CO3��CO2Ϊ��Ӧ�Ļ�����������ѡ��Ӧ��Ƴ�һ��ԭ��أ���д����ԭ��صĸ�����Ӧ��_____________________________________��
��2��ijʵ��С��ģ�ҵ�ϳɰ���ӦN2��g����3H2��g�� 2NH3��g������H����92.4 kJ��mol��1����ʼ���ǽ�N2��H2�������20 mol�������1��1������5 L�ϳ����У���ӦǰѹǿΪP0����Ӧ������ѹǿ��P��ʾ����Ӧ������
2NH3��g������H����92.4 kJ��mol��1����ʼ���ǽ�N2��H2�������20 mol�������1��1������5 L�ϳ����У���ӦǰѹǿΪP0����Ӧ������ѹǿ��P��ʾ����Ӧ������ ��ʱ��t�Ĺ�ϵ��ͼ��ʾ��
��ʱ��t�Ĺ�ϵ��ͼ��ʾ��
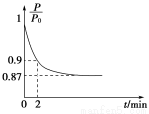
��ش��������⣺
����Ӧ��ƽ��ı�־��__________________________������ĸ���ţ���ͬ����
A��ѹǿ���ֲ���
B�������ܶȱ��ֲ���
C��NH3������������N2���������ʵ�2��
��0��2 min�ڣ���c��N2���仯��ʾ��ƽ����Ӧ����Ϊ________________��
�������N2��ת���ʣ��ɲ�ȡ�Ĵ�ʩ��_____________________________��
A������ϵ�а������1��1�ٳ���N2��H2
B�������NH3
C�������¶�
D�����뺤��ʹѹǿ����
E������һ������N2
��3��25��ʱ��BaCO3��BaSO4���ܶȻ������ֱ���8��10��9��1��10��10��ij����BaCO3����������Һ�У�c��CO32-����0.2 mol��L��1���������������Na2SO4��Һ����Ҫ����BaSO4����������Na2SO4��Һ�����ʵ���Ũ����С��________mol��L��1��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014��߿���ѧ���ָ�ϰר�������2 ��ѧ����������ϰ���������棩 ���ͣ�ѡ����
�����йػ�ѧʵ������������ǣ�������
��������FeSO4��Һʱ�������м���һ�������ۺ�ϡ����
������100 mL 1.00 mol/L��NaCl��Һʱ������������ƽ��ȡ5.85 g NaCl����
����������ˮ�Ĺ����У�һ���з��Ȼ�������������ܽ�IJ�����������Ͳ�н���
�����Թܼд��Թܵ��������ϼ�ס���Թܿ�Լ1/3�����ֳ��Թܼг���ĩ�ˣ����м���
��������Ũ����մ��Ƥ���ϣ�������NaHCO3��Һ��ϴ
���ù㷺pH��ֽ���ij��Һ��pH��12.3
���ü�ʽ�ζ�����ȡ20.00 mL 0.100 0 mol/L KMnO4��Һ
����ͭ������������ͭ���������ᷴӦ����ȡ����ͭ
A���ܢݢޢ� B���٢ܢݢ� C���ڢۢޢ� D���٢ۢܢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014��߿���ѧ���ָ�ϰר�������1 ��ѧ����������ϰ���������棩 ���ͣ�ѡ����
ʵ�������Ʊ���ϩ�ķ�����ʹ�Ҵ���ˮ����Ӧ���Լر�ʾΪCH3CH2OH�D��CH2=CH2����H2O����֪CH2=CH2��g����C2H5OH��l����ȼ���ȷֱ���1 411.0 kJ��mol��1��1 366.8 kJ��mol��1����ʵ������C2H5OH��l���Ʊ�CH2=CH2��g������1 molҺ̬ˮ����HΪ��������
A����44.2 kJ��mol��1
B����44.2 kJ��mol��1
C����2 777.8 kJ��mol��1
D����2 777.8 kJ��mol��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014��߿���ѧ���ָ�ϰר�������1 ��ѧ����������ϰ���������棩 ���ͣ�ѡ����
���л�ѧ�����ʾ��ȷ���ǣ�������
A��Cl���Ľṹʾ��ͼ��
B��������ӵ����ģ�ͣ�
C���������Ƶĵ���ʽ��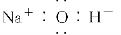
D��������Ľṹʽ��H��O��Cl
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014��߿���ѧһ�ָ�ϰ�κ�ѵ��ר����ϰ�����������棩 ���ͣ�ѡ����
���������У���ȷ���ǣ� ��
A���Ҵ�������������������ñ���Na2CO3��Һ����
B������ϩ�ɷ����ӳɷ�Ӧ
C�����е����ࡢ��֬�͵����ʶ��ܷ���ˮ�ⷴӦ
D�����Ϸ���ʽC5H12��������4��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014��߿���ѧһ�ָ�ϰ�κ�淶ѵ��9-2��ϰ���������棩 ���ͣ�ѡ����
�Ը����Ϊԭ�ϣ��绯ѧ���Ʊ��ظ���ص�ʵ��װ��ʾ��ͼ���£�
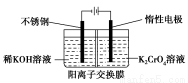
����˵������ȷ���ǣ� ��
A���������ң������ĵ缫��ӦΪ��2H2O��2e��=2OH����H2��
B���������ң�ͨ�����Һ���ɻ�ɫ��Ϊ��ɫ������Ϊ������H��Ũ������ʹƽ��2CrO42-��2H�� Cr2O72-��H2O�����ƶ�
Cr2O72-��H2O�����ƶ�
C�����Ʊ������ܷ�Ӧ�Ļ�ѧ����ʽΪ��4K2CrO4��4H2O 2K2Cr2O7��4KOH��2H2����O2��
2K2Cr2O7��4KOH��2H2����O2��
D���ⶨ����Һ��K��Cr�ĺ�������K��Cr�����ʵ���֮��(nk/nCr)Ϊd�����ʱ����ص�ת����Ϊ1��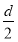
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com