
����������Ԫ��A��B��C��D��E��ԭ�����������������ǵ�ԭ�Ӻ������֮��Ϊ10��B�Ļ���������࣬��Ŀ�Ӵ�C��D�ǿ����к�����������Ԫ�أ�D��E�����ʿ����������ֲ�ͬ�����ӻ����
��1��д��E�ĵ�����A��D��Ԫ���γ��䳣�������ﷴӦ�����ӷ���_________________��
��2����A��C��D��Ԫ�����γɵij�������Һ��_________�ԣ���ᡱ�����С������������ԭ�������ӷ���ʽ��ʾΪ��__________________________________________��
��3��B����Է���������С���⻯���ȼ����Ϊ890.3kJ?mol��1��д����ȼ��ʱ���ȷ���ʽ____________________��
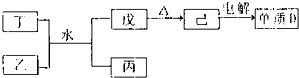
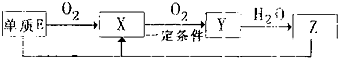
![]() ��4��X�Ǻ���A��C��Ԫ�صĻ�����ڹ�ҵ����X�Ϳ������������������������Ϊ0.2��Ϊԭ��������Z�Ĺ������£�
��4��X�Ǻ���A��C��Ԫ�صĻ�����ڹ�ҵ����X�Ϳ������������������������Ϊ0.2��Ϊԭ��������Z�Ĺ������£�
![]()

��a��������з�����������Ӧ������������ѧ����ʽ�ϲ�Ϊһ����ѧ����ʽ���ɱ�ʾΪ________________________��
��b���������Ǹ���Ӧ�Ҹ�����Ӧ����ȫ��Ϊʹ������Z�Ĺ����в��ٲ����������ԭ������X������������ֵΪ���٣���Ҫ��д��������̣�
��1��2Na+2H2O��2Na++2OH��+H2����2�֣�
��2���ᣨ1�֣�NH4++H2O![]() NH3?H2O+H+��2�֣�
NH3?H2O+H+��2�֣�
��3��CH4��g��+2O2��g����CO2��g��+2H2O��l������H=��890.3KJ/mol��2�֣�
��4����4NO+3O2+2H2O��4HNO3��2�֣�
��1/6��16.7%��4�֣���Ҫ����̣�
�⣺����ԭ����Ϊ1L,����NH3���������ΪX (1��)
���йط�Ӧ����ʽ�ã�
NH3 ![]() 2O2
2O2 ![]() HNO3
HNO3![]() NH4NO3
NH4NO3![]() NH3(�����ᷴӦ��)
NH3(�����ᷴӦ��)
�������е�O2��ȫ����ʱNH3���������X���
�� 2 NH3 ![]() 2O2
2O2
1 1
1L��X 1L(1�DX)��0.2 (1��)
 X=1/6 (1��)
X=1/6 (1��)
��ԭ������NH3������������ֵΪ1/6 (1��)


 �����������Ů��ͯ������ϵ�д�
�����������Ů��ͯ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||
| ||
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ����������Ԫ��A��B��C��D��E��ԭ������������������ԭ�Ӻ���ĵ��Ӳ���֮��Ϊ10��BԪ�صĻ���������࣬��Ŀ�Ӵ�C��D����Ԫ���γɵĵ����ǿ����к����������ʣ�D��E��Ԫ�ؿ����������ֲ�ͬ�����ӻ����
����������Ԫ��A��B��C��D��E��ԭ������������������ԭ�Ӻ���ĵ��Ӳ���֮��Ϊ10��BԪ�صĻ���������࣬��Ŀ�Ӵ�C��D����Ԫ���γɵĵ����ǿ����к����������ʣ�D��E��Ԫ�ؿ����������ֲ�ͬ�����ӻ���� NH3?H2O+H+
NH3?H2O+H+ NH3?H2O+H+
NH3?H2O+H+ 2NO2
2NO2 2NO2
2NO2| 1 |
| 3 |
| 1 |
| 3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��ԭ�Ӱ뾶��A��D��C��B | B��B��C��D�ֱ���A�γɵĻ�����һ��������ͬ�Ļ�ѧ�� | C������������Ӧˮ��������ԣ�D��C | D�������£�����B�ܴ�������Ũ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��ԭ�ڰ뾶A��B��C | B��A����̬�⻯���ȶ��Դ���C����̬�⻯���ȶ��� | C��A��C��Ԫ����������������ˮ���ϵõ���Ӧ���� | D������ʱ��A���ʿ��Դ�C�����������û��õ�C���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��ԭ�Ӱ뾶��С�����˳��r��C����r��D����r��E�� | B��Ԫ��D��E�ֱ���A�γɵĻ���������ȶ��ԣ�E��D | C��Ԫ��D������������Ӧˮ��������Ա�E��ǿ | D��Ԫ��B�ֱ���A��C�γɵĻ������л�ѧ����������ȫ��ͬ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com