
��6�֣�ʵ������ȡ������������Ҫ�������£�

���ڼ��Թܣ���ͼ���м���2mLŨ���ᡢ3mL�Ҵ���2mL����Ļ����Һ��
�ڰ���ͼ���Ӻ�װ�ã�װ�����������ã���������Һ����С����ȵؼ���3��5min��
�۴��Թ����ռ���һ���������ֹͣ���ȣ������Թ��Ҳ�������Ȼ���ô��ֲ㡣�ܷ�������������㡢ϴ�ӡ����
��1�����Ƹû����Һ����Ҫ��������Ϊ��_______________________________________ ����Ӧ��Ũ�����������___________________д����ȡ���������Ļ�ѧ����ʽ��___________________________________��
��2������ʵ���б���̼������Һ�������ǣ�����ĸ����_______________��
A���к�������Ҵ���
B���к����Ტ���ղ����Ҵ���
C�����������ڱ���̼������Һ�е��ܽ�ȱ���ˮ�и�С�������ڷֲ�������
��3���������Թ��е����ʷ��뿪�Եõ���������������ʹ�õ������� __________������ʱ����������Ӧ�ô����� ________ ������¿ڷš� ���Ͽڵ���������
 ���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д�
���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д� ����ѵ��ϵ�д�
����ѵ��ϵ�д� ��ĩ�����ϵ�д�
��ĩ�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ij����С����Ƶ�ʵ������ȡ����������װ����ͼ��ʾ��A�з���Ũ���ᣬB�з����Ҵ�����ˮ�����ƣ�D�з��б���̼������Һ��
ij����С����Ƶ�ʵ������ȡ����������װ����ͼ��ʾ��A�з���Ũ���ᣬB�з����Ҵ�����ˮ�����ƣ�D�з��б���̼������Һ��| �Լ� | ���� | �Ҵ� | ���� | �������� |
| �е�/�� | 34.7 | 78.5 | 118 | 77.1 |
| Ũ���� |
| �� |
| Ũ���� |
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ʵ������ȡ����������ʵ��װ����ͼ��ʾ����ش��������⣮
ʵ������ȡ����������ʵ��װ����ͼ��ʾ����ش��������⣮| ŨH2SO4 |
| �� |
| ŨH2SO4 |
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
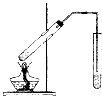 ʵ��������ͼ��ʾװ����ȡ����������
ʵ��������ͼ��ʾװ����ȡ����������| Ũ���� |
| ���� |
| Ũ���� |
| ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A�������ʯ��������Ϊ�˷�ֹҺ�屩�� | B�������¶Ȳ��˹�����Ϊ�˷�ֹ�Ҵ�������Ļӷ� | C��������ռ��ڱ���Na2CO3��Һ�Ϸ�����Ϊ�˳�ȥ���������е��Ҵ��ʹ��� | D����Ӧ�������������ԭ�ӣ�-H�����Ҵ����ǻ���-OH�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��ͼΪʵ������ȡ����������װ��ͼ���Իش�
��ͼΪʵ������ȡ����������װ��ͼ���Իش��鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com