
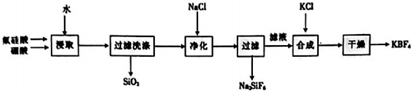
 £»
£»·ÖĪö ŅŌ·ś¹čĖį£ØH2SiF4£©”¢ÅšĖį[H3BO4]µČĪŖŌĮĻÖʱø·śÅšĖį¼Ų£¬ÓÉĮ÷³ĢæÉÖŖ£¬H2SiF4”¢H3BO4ÖŠ¼ÓĖ®½žČ”£¬Éś³ÉHBF4ŗĶSiO2£¬¹żĀĖ³żČ„SiO2£¬ĀĖŅŗÖŠ¼ÓĀČ»ÆÄĘ£¬Éś³ÉNa2SiF4³Įµķ£¬½ųŅ»²½³żČ„ČÜŅŗÖŠŗ¬ÓŠÉŁĮæH2SiF4£¬¹żĀĖĀĖµĆHBF4ČÜŅŗ£¬ĀĖŅŗÖŠ¼ÓĀČ»Æ¼ŲÉś³ÉKBF4³Įµķ£¬¾¹żĀĖ”¢Ļ“µÓ”¢øÉŌļŗóµĆ¹ĢĢåKBF4£¬
£Ø1£©H2SiF4ĪŖ¶žŌŖĒæĖį£¬¾Ż“Ė“šĢā£»
£Ø2£©H3BO4ĪŖŅ»ŌŖČõĖį£¬ĖįøłĪŖB£ØOH£©4-£¬ĖłŅŌÅšĖįµēĄėŹ±ŅŖ½įŗĻŅ»·Ö×ÓĖ®£¬øł¾ŻµēŗÉŹŲŗćŹéŠ“µēĄė·½³ĢŹ½£»
£Ø3£©·śÅšĖį¼Ų£ØKBF4£©ÖŠŗ¬ÓŠĄė×Ó¼üŗĶ¹²¼Ū¼ü”¢ÅäĪ»¼ü£»
£Ø4£©øł¾ŻŌŖĖŲŹŲŗćŹéŠ“»Æѧ·½³ĢŹ½£¬øł¾ŻÓ°Ļģ·“Ó¦ĖŁĀŹµÄŅņĖŲÅŠ¶Ļ²ÉČ”µÄ“ėŹ©£»
£Ø5£©¼ÓĀČ»ÆÄĘ£¬Éś³ÉNa2SiF4³Įµķ£¬æÉŅŌ½ųŅ»²½³żČ„ČÜŅŗÖŠŗ¬ÓŠÉŁĮæH2SiF4£»
£Ø6£©¢ŁBF3ÖŠĆæøöÅšŗĶ·śŠĪ³ÉŅ»¶Ō¹²ÓƵē×Ó¶Ō£»
¢Śøł¾ŻŌŖĖŲŹŲŗć£¬½įŗĻŹ½ĮæÅŠ¶Ļ²śĪļ£»
£Ø7£©ŌŚ¼ÓČČĢõ¼žĻĀ£¬·ś»Æļ§ÓėK2O•B2O3ÖʵƷśÅšĖį¼Ų£¬ĮķĶāÓŠ°±ĘųŗĶĖ®²śÉś¾Ż“ĖŹéŠ“»Æѧ·½³ĢŹ½£®
½ā“š ½ā£ŗŅŌ·ś¹čĖį£ØH2SiF4£©”¢ÅšĖį[H3BO4]µČĪŖŌĮĻÖʱø·śÅšĖį¼Ų£¬ÓÉĮ÷³ĢæÉÖŖ£¬H2SiF4”¢H3BO4ÖŠ¼ÓĖ®½žČ”£¬Éś³ÉHBF4ŗĶSiO2£¬¹żĀĖ³żČ„SiO2£¬ĀĖŅŗÖŠ¼ÓĀČ»ÆÄĘ£¬Éś³ÉNa2SiF4³Įµķ£¬½ųŅ»²½³żČ„ČÜŅŗÖŠŗ¬ÓŠÉŁĮæH2SiF4£¬¹żĀĖĀĖµĆHBF4ČÜŅŗ£¬ĀĖŅŗÖŠ¼ÓĀČ»Æ¼ŲÉś³ÉKBF4³Įµķ£¬¾¹żĀĖ”¢Ļ“µÓ”¢øÉŌļŗóµĆ¹ĢĢåKBF4£¬
£Ø1£©H2SiF4ĪŖ¶žŌŖĒæĖį£¬ĖłŅŌ³£ĪĀĻĀ£¬0.05mol•L-1 H2SiF4ČÜŅŗÖŠĒāĄė×ÓÅضČĪŖ0.1mol•L-1£¬ĖłŅŌpHĪŖ1£¬
¹Ź“š°øĪŖ£ŗ1£»
£Ø2£©H3BO4ĪŖŅ»ŌŖČõĖį£¬ĖįøłĪŖB£ØOH£©4-£¬ĖłŅŌÅšĖįµÄµēĄė·½³ĢŹ½ĪŖH2O+H3BO4 ?B£ØOH£©4-+H+£¬
¹Ź“š°øĪŖ£ŗH2O+H3BO4 ?B£ØOH£©4-+H+£»
£Ø3£©·śÅšĖį¼Ų£ØKBF4£©ÖŠĖłŗ¬»Æѧ¼üµÄĄąŠĶĪŖĄė×Ó¼üŗĶ¹²¼Ū¼ü”¢ÅäĪ»¼ü£¬
¹Ź“š°øĪŖ£ŗĄė×Ó¼üŗĶ¹²¼Ū¼ü”¢ÅäĪ»¼ü£»
£Ø4£©½žČ”Ź±Éś³ÉHBF4µÄ»Æѧ·½³ĢŹ½ĪŖH2SiF4+H3BO4=HBF4+SiO2”ż+2H2O£¬ÓÉÓŚĖį½žŹ±æóŹÆæÅĮ£“óŠ”Ó°Ļģ½žČ”ĀŹ£¬æÉŅŌĶعż·ŪĖé¹ĢĢåĢįøß½žČ”ĀŹ£¬»¹æÉŅŌŹŹµ±ÉżøßĪĀ¶Č»ņÕß½Į°čĢįøß½žČ”ĀŹ£¬
¹Ź“š°øĪŖ£ŗH2SiF4+H3BO4=HBF4+SiO2”ż+2H2O£»·ŪĖé¹ĢĢ唢ŹŹµ±ÉżøßĪĀ¶Č»ņÕß½Į°čµČ£»
£Ø5£©¼ÓĀČ»ÆÄĘ£¬Éś³ÉNa2SiF4³Įµķ£¬½ųŅ»²½³żČ„ČÜŅŗÖŠŗ¬ÓŠÉŁĮæH2SiF4£¬
¹Ź“š°øĪŖ£ŗ³żČ„ČÜŅŗÖŠŗ¬ÓŠÉŁĮæH2SiF4£»
£Ø6£©¢ŁBF3µÄµē×ÓŹ½ĪŖ £¬
£¬
¹Ź“š°øĪŖ£ŗ £»
£»
¢Ś½«BF3ÓėKBH4ŅŌĪļÖŹµÄĮæÖ®±Č4£ŗ3Ē”ŗĆĶźČ«·“Ó¦ÖʵƷśÅšĖį¼ŲŗĶŅ»ÖÖĘųĢ壬ĘųĢå²śĪļ£ØĦ¶ūÖŹĮæ½éÓŚ20”«30g•mol-1£©£¬øł¾ŻŌŖĖŲŹŲŗćæÉÖŖøĆĘųĢåµÄ»ÆѧŹ½ĪŖB2H6£¬
¹Ź“š°øĪŖ£ŗB2H6£»
£Ø7£©ŌŚ¼ÓČČĢõ¼žĻĀ£¬·ś»Æļ§ÓėK2O•B2O3Ņ²æÉÖʵƷśÅšĖį¼Ų£¬øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ8NH4F+K2O•B2O3$\frac{\underline{\;¼ÓČČ\;}}{\;}$2KBF4+8NH3”ü+4H2O£¬
¹Ź“š°øĪŖ£ŗ8NH4F+K2O•B2O3$\frac{\underline{\;¼ÓČČ\;}}{\;}$2KBF4+8NH3”ü+4H2O£®
µćĘĄ ±¾Ģāæ¼²é»ģŗĻĪļ·ÖĄėĢį“æµÄ×ŪŗĻÓ¦ÓĆ£¬ĪŖøßĘµæ¼µć£¬ĪŖ2015Äźøßæ¼ÕęĢā£¬°ŃĪÕŹµŃéĮ÷³Ģ¼°·¢ÉśµÄ·“Ó¦”¢»ģŗĻĪļ·ÖĄėĢį“æ·½·ØĪŖ½ā“šµÄ¹Ų¼ü£¬²ąÖŲ·ÖĪöÓėŹµŃéÄÜĮ¦µÄ×ŪŗĻ漲飬ĢāÄæÄѶČÖŠµČ£®


 ¾ŁŅ»·“Čżµ„ŌŖĶ¬²½¹ż¹Ų¾ķĻµĮŠ“š°ø
¾ŁŅ»·“Čżµ„ŌŖĶ¬²½¹ż¹Ų¾ķĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2016-2017ѧğ°²»ÕŹ”øßŅ»ÉĻµŚŅ»“ĪŌĀæ¼»Æѧ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŃ”ŌńĢā
ÓūÅäÖĘ100mL 1.0 mol/L Na2CO3ČÜŅŗ£¬ÕżČ·µÄ·½·ØŹĒ
¢Ł ½«10.6 g Na2CO3 ČÜÓŚ100mLĖ®ÖŠ
¢Ś ½«28.6g Na2CO3”¤10H2OČÜÓŚÉŁĮæĖ®ÖŠ£¬ŌŁÓĆĖ®Ļ”ŹĶÖĮ100 mL
¢Ū ½«20 ml 5.0 mol/L Na2CO3ČÜŅŗÓĆĖ®Ļ”ŹĶÖĮ100 mL
A£®¢Ł¢Ś B£®¢Ś¢Ū C£®Ö»ÓŠ¢Ś D£®Ö»ÓŠ¢Ū
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2017½ģ½ĖÕŹ”øßČżÉĻµŚŅ»“Ī²āŹŌ»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗ¼ĘĖćĢā
¼īŹ½Ģ¼ĖįÄĘĀĮ[NaaAlb(OH)c(CO3)d]æÉÓĆ×÷×čČ¼¼Į”¢æ¹Ėį¼ĮµČ”£
ĘäÖʱø·½·ØŹĒ£ŗæŲÖĘĪĀ¶Č”¢pH£¬ĻņNaHCO3Ļ”ČÜŅŗÖŠ¼ÓČėAl(OH)3£¬²¢½Į°č£¬³ä·Ö·“Ó¦ŗó¹żĀĖ”¢Ļ“µÓ”¢øÉŌļ£¬µĆ¼īŹ½Ģ¼ĖįÄĘĀĮ”£
£Ø1£© ¼īŹ½Ģ¼ĖįÄĘĀĮ[NaaAlb(OH)c(CO3)d]ÖŠa”¢b”¢c”¢dÖ®¼äµÄ¹ŲĻµĪŖ____________”£
£Ø2£© ¼īŹ½Ģ¼ĖįÄĘĀĮ×÷ĪŖ×čČ¼¼ĮµÄæÉÄÜŌŅņ£ŗ¢ŁŌŚ·Ö½ā¹ż³ĢÖŠ“óĮæĪüČČ£»¢Ś±¾Éķ¼°²śĪļĪŽ¶¾ĒŅ²»æÉČ¼£»
¢Ū ”£
£Ø3£© ČōpH¹żøߣ¬Ōņ¶Ō²śĘ·µÄÓ°ĻģŹĒ ”£
£Ø4£© ĪŖČ·¶Ø¼īŹ½Ģ¼ĖįÄĘĀĮµÄ×é³É£¬½ųŠŠČēĻĀŹµŃé£ŗ
¢Ł×¼Č·³ĘČ”2.880 gѳʷÓĆ×ćĮæĻ”ĻõĖįČܽā£¬µĆµ½CO2 0.448 L(ŅŃ»»Ėć³É±ź×¼×“æöĻĀ)”£ŌŚĖłµĆČÜŅŗÖŠ¼Ó¹żĮæ°±Ė®£¬µĆµ½°×É«³Įµķ£¬¾¹żĀĖ”¢Ļ“µÓ³Įµķ”¢³ä·Ö×ĘÉÕµĆµ½1.02g¹ĢĢ唣
¢Ś¼ÓČČÖĮ340 ”ęŅŌÉĻŹ±ŃłĘ·ŃøĖŁ·Ö½ā£¬µĆµ½½šŹōŃõ»ÆĪļ”¢CO2ŗĶH2O”£µ±ŃłĘ··Ö½āĶźČ«Ź±£¬ŃłĘ·µÄ¹ĢĢ岊ĮōĀŹĪŖ56.9%£¬øł¾ŻŅŌÉĻŹµŃ鏿¾ŻČ·¶Ø¼īŹ½Ģ¼ĖįÄĘĀĮµÄ×é³É(Š“³ö¼ĘĖć¹ż³Ģ)”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
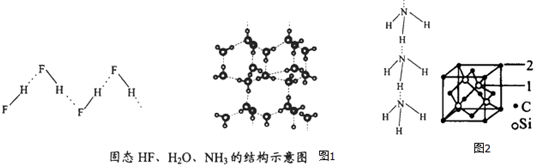
| ĪļÖŹ | Ēā¼üX-H”Y | ¼üÄÜkJ£®mol-1 |
| £ØHF£©n | D-H”F | 28.1 |
| ±ł | O-H”O | 18.8 |
| £ØNH3£©n | N-H”N | 5.4 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
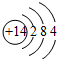 £®
£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ĻņĮņĖįĀĮČÜŅŗÖŠ¼ÓČėÉŁĮæµÄ°±Ė®£ŗAl3++3OH-ØTAl£ØOH£© 3”ż | |
| B£® | ĻņĢ¼ĖįÄĘČÜŅŗÖŠ¼ÓČė±„ŗĶŹÆ»ŅĖ®£ŗCa£ØOH£©2+CO32-ØTCaCO3”ż+2OH- | |
| C£® | ĻņĒāŃõ»ÆÄĘČÜŅŗÖŠĶØČė¹żĮæµÄĀČĘų£ŗCl2+2OH-ØTClO-+Cl-+H2O | |
| D£® | ĻņĻ”ĮņĖįÖŠ¼ÓČė¹żĮæĢś·Ū£¬ĻČŗó·¢ÉśĮ½øö·“Ó¦£ŗ2Fe+6H+ØT2Fe3++3H2”ü£¬Fe+2Fe3+ØT3Fe2+ |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com