
( 10��)��84����Һ����������Һ��Ӧ������ȡ������NaClO+ NaCl+H2SO4 Na2SO4 + Cl2��+H2O�� Ϊ̽�����������ʣ�ijͬѧ���ô�ԭ���������������������ʾ��ʵ��װ��
Na2SO4 + Cl2��+H2O�� Ϊ̽�����������ʣ�ijͬѧ���ô�ԭ���������������������ʾ��ʵ��װ��

��1���Ӣ١��ڡ���װ����ѡ����ʵ�����װ�ã�A����___________ (��д���)��

��2��װ��B��C�����ηŵ��Ǹ���ĺ�ɫ������ʪ��ĺ�ɫ������ʵ������и�ͬѧ����װ��B�еIJ���Ҳ��ɫ����ԭ�������__________˵����װ�ô������Ե�ȱ�ݣ�����������ĸĽ��ķ��� _______________________________
��3��Ϊ����֤�����������ԣ�������ͨNa2SO3��Һ�У�д��������Na2SO3��Һ��Ӧ�����ӷ���ʽ ______________________________________
��4������ͨ�뱥��NaHCO3��Һ�ܲ�����ɫ���壬��֪����:����>̼��>�����ᣬ��ʵ��֤��������ˮ��Ӧ���������к��� __________________________
��1���� ��2�֣���2�������������к���ˮ�����ᷴӦ���ɴ����ᣨ2�֣�
��װ��A��B֮������ʢ��Ũ�����ϴ��ƿ�������װ�ã���2�֣�
��3��Cl2 + SO32��+ H2O = SO42�� + 2Cl��+ 2H+��2�֣� ��4��HCl�������ᣩ��2�֣�
��������
�����������1��������ȡ�����ķ���ʽ��֪���÷�Ӧ�ǹ����Һ�巴Ӧ��ȡ����ģ����ѡ���װ��Ӧ���Ǣڡ�
��2�����ڲ����������к���ˮ���������������Ӧ���ɴ����ᣬ���������Ư���ԣ�ʹ������ɫ�����Ҫ��ֹ������ķ���������Ҫ�������������Ժ����ĸĽ���������װ��A��B֮������ʢ��Ũ�����ϴ��ƿ�������װ�ã���
��3�������ܰ����������������������ƣ���Ӧ�����ӷ���ʽ��Cl2 + SO32��+ H2O = SO42�� + 2Cl��+ 2H+��
��4����������ǿ��˳�������̼������ᣬ���Ը��ݽ�ǿ������ȡ���������Լ����ɵ�CO2��֪����Ӧ��һ�����������ɣ�������ȷ���Ƿ��д��������ɡ�
���㣺�����������Ʊ����������ʼ����
������������Ҫ��Χ�����������Ʊ������ʼ��飬�ص㿼��ѧ����ʵ����������Ĺ淶�Ժ�ȷ�ԣ����������֪ʶ���ʵ�������������


 ��ѧ��������������Ͼ���ѧ������ϵ�д�
��ѧ��������������Ͼ���ѧ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2013�����ʡ����ʮУ������һ�����ϼ�⻯ѧ�Ծ����������� ���ͣ������
( 10��) ��84����Һ����������Һ��Ӧ������ȡ������NaClO+ NaCl+H2SO4 Na2SO4 + Cl2��+H2O��Ϊ̽�����������ʣ�ijͬѧ���ô�ԭ���������������������ʾ��ʵ��װ��

��ش�
��1���Ӣ١��ڡ���װ����ѡ����ʵ�����װ�ã�A���� (��д���)��
��2��װ��B��C�����ηŵ��Ǹ���ĺ�ɫ������ʪ��ĺ�ɫ������ʵ������и�ͬѧ����װ��B�еIJ���Ҳ��ɫ����ԭ������� ��˵����װ�ô������Ե�ȱ�ݣ�����������ĸĽ��ķ��� ��
��3��Ϊ����֤�����������ԣ�������ͨNa2SO3��Һ�У�д��������Na2SO3��Һ��Ӧ�����ӷ���ʽ ��
��4������ͨ�뱥��NaHCO3��Һ�ܲ�����ɫ���壬��֪��������>̼��>�����ᣬ��ʵ��֤��������ˮ��Ӧ���������к��� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��ӱ�ʡ�ݳ���ѧ������ѧ�ڵ������¿���ѧ�Ծ����������� ���ͣ�ʵ����
( 10��)��84����Һ����������Һ��Ӧ������ȡ������NaClO+ NaCl+H2SO4 Na2SO4 + Cl2��+H2O��Ϊ̽�����������ʣ�ijͬѧ���ô�ԭ���������������������ʾ��ʵ��װ��
Na2SO4 + Cl2��+H2O��Ϊ̽�����������ʣ�ijͬѧ���ô�ԭ���������������������ʾ��ʵ��װ��
��1���Ӣ١��ڡ���װ����ѡ����ʵ�����װ�ã�A����___________ (��д���)��
��2��װ��B��C�����ηŵ��Ǹ���ĺ�ɫ������ʪ��ĺ�ɫ������ʵ������и�ͬѧ����װ��B�еIJ���Ҳ��ɫ����ԭ�������__________˵����װ�ô������Ե�ȱ�ݣ�����������ĸĽ��ķ���_______________________________
��3��Ϊ����֤�����������ԣ�������ͨNa2SO3��Һ�У�д��������Na2SO3��Һ��Ӧ�����ӷ���ʽ ______________________________________
��4������ͨ�뱥��NaHCO3��Һ�ܲ�����ɫ���壬��֪����:����>̼��>�����ᣬ��ʵ��֤��������ˮ��Ӧ���������к��� __________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�����ʡ����ʮУ������һ�����ϼ�⻯ѧ�Ծ��������棩 ���ͣ������
( 10��) ��84����Һ����������Һ��Ӧ������ȡ������NaClO+ NaCl+H2SO4 Na2SO4 + Cl2��+H2O��Ϊ̽�����������ʣ�ijͬѧ���ô�ԭ���������������������ʾ��ʵ��װ��


��ش�
��1���Ӣ١��ڡ���װ����ѡ����ʵ�����װ�ã�A���� (��д���)��
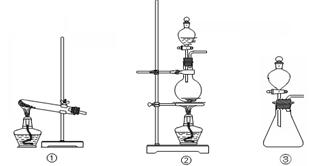
��2��װ��B��C�����ηŵ��Ǹ���ĺ�ɫ������ʪ��ĺ�ɫ������ʵ������и�ͬѧ����װ��B�еIJ���Ҳ��ɫ����ԭ������� ��˵����װ�ô������Ե�ȱ�ݣ�����������ĸĽ��ķ��� ��
��3��Ϊ����֤�����������ԣ�������ͨNa2SO3��Һ�У�д��������Na2SO3��Һ��Ӧ�����ӷ���ʽ ��
��4������ͨ�뱥��NaHCO3��Һ�ܲ�����ɫ���壬��֪��������>̼��>�����ᣬ��ʵ��֤��������ˮ��Ӧ���������к��� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(10��) �ش���������
��1��д�����������봿����Һ��Ӧ�Ļ�ѧ����ʽ��_______________________________________________��
��2���ݱ�������ȫ�����ط����˶���84����Һ��������ʹ�ã����������ж����¼���д���÷�Ӧ�����ӷ�Ӧ����ʽ��_____________________________________________________________________��
��3���������������ʵ���������Ӧ������ˮ����һ��������������������Ϊ_______________��
��4����������أ�K2S2O8������ǿ�����ԣ��ɽ�I������ΪI2��S2O82����2I����2SO42����I2����֪Fe3+�����Ա�S2O82������������Fe2+�Ļ�ԭ�Ա�I����ԭ������ʵ��ȴ����Fe3+��Fe2+�ɴ�������Ӧ������Fe3+�ܼӿ�÷�Ӧ����������õ�ԭ��________________________________________________________________________________��
��5���л������У��ļ۵�̼ԭ����һ�����������������ĸ������ֱ�����һ�����������������ĸ�����̼ԭ��ʱ�����ֱ��Ϊ�����١����̼ԭ�ӣ�Ҳ���Էֱ��Ϊ��һ���ڶ������������̼ԭ�ӣ�������2,2,4-������������5����̼ԭ�ӣ��١��塢��̼ԭ�Ӹ�1������6����̼ԭ�Ӻ�6����̼ԭ�ӣ�����Ϊ��ԭ�ӣ����ɵı������� �֣�������˳���칹��ѧ�칹����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com