
Ŀǰ������60%��þ�ǴӺ�ˮ����ȡ�ġ���֪��ˮ��ȡþ����Ҫ�������£�

��1�����ڼ����Լ������������������¼��ֲ�ͬ������������������⡣
���� | �Ƿ���ȷ | �������� |
����1��ֱ������ˮ�м�������� | ����ȷ | ��һ�� |
����2�����¼���������ˮ���ټ�������� | ������ | ������ |
����Ϊ����������������ǣ����������ģ� | ||
��һ��_______________________________________________��
������_______________________________________________��
������______________________________________________��
���ģ�______________________________________________��
��2����ͼ�м�����Լ���Ӧ����________���ѧʽ����������Լ�����________���ѧʽ������ҵ������ˮMgCl2��ȡþ�Ļ�ѧ����ʽΪ________________________________________��
��1����һ����ˮ��þ����Ũ��С������������������
����������ȷ
��������Դ���Ĵ�����
���ģ���̲ɹ�κ�õ��Ŀ�±ˮ�У����������
��2��Ca��OH��2��HCl��MgCl2�����ڣ�  Mg��Cl2��
Mg��Cl2��
�����������⿼����ǴӺ�ˮ����ȡþ�����̡��Լ���Ӧ��ʯ���飬�����ķ�Ӧ��MgCl2��Ca��OH��2=Mg��OH��2����CaCl2���Լ���Ӧ�����ᣬ��Ӧ��Mg��OH��2��2HCl=MgCl2��2H2O��Ȼ��Ũ�����ᾧ����ˮ����ˮMgCl2���ٵ�����ڵ�MgCl2����Ƶ�Mg��MgCl2 �����ڣ�  Mg��Cl2����
Mg��Cl2����


 �ִʾ�ƪ��ͬ�����Ĵ��ϵ�д�
�ִʾ�ƪ��ͬ�����Ĵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2014����л�ѧ���ִ���ѵ���� ר��14���ʽṹ������ѡ����ϰ���������棩 ���ͣ������
�ڵ�����������м������ʯ������A�����棩��������Al2O3�۵�����á�����ʯ������ԭ��Ϊ2Al��OH��3��12HF��3Na2CO3=2A��3CO2����9H2O�������������������գ�
��1������ʯ�Ļ�ѧʽΪ________���������Ӽ���________�Ȼ�ѧ����
��2���������к���10�����ӵķ�����________��д����ʽ�����÷��ӵĿռ乹��Ϊ________������ԭ�ӵ��ӻ���ʽΪ________��
��3����Ӧ���е縺������Ԫ��Ϊ________����Ԫ�ط��ţ���д����ԭ��������ĵ����Ų�ͼ��________��
��4��Al���ʵľ�����ԭ�ӵĶѻ���ʽ��ͼ����ʾ���侧��������ͼ����ʾ��ԭ��֮���λ�ù�ϵ��ƽ��ͼ��ͼ����ʾ��

����֪Al��ԭ�Ӱ뾶Ϊd��NA���������ӵ�������Al�����ԭ������ΪM����һ��������Alԭ�ӵ���ĿΪ________��Al������ܶ�Ϊ________������ĸ��ʾ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014���һ��ѧ�˽̰����1-2-3Ԫ�����ڱ�Ԫ��������Ӧ����ϰ���������棩 ���ͣ������
X��Y��Z��W��ԭ��������������Ķ�����Ԫ�أ��һ���ͬ�壻����ֻ������Ϊ������Xԭ�ӵ������������������������ȣ�X��W��Y��Z������ԭ�ӵ�����������֮�;�Ϊ9������Y��W������Ũ��NaOH��Һ��Ӧ����ش��������⣺
��1��Y��Z��W��ԭ�Ӱ뾶��С�����˳����________����Ԫ�ط��ű�ʾ����
��2��ZW2�ķ���ʽΪ________________��
��3����ҵ��������Y��ԭ����__________________���û�ѧ����ʽ��ʾ����
��4��X��Y��ѧ�������ƣ���X��Ũ��NaOH��Һ��Ӧ�Ļ�ѧ����ʽ��___________________________________________________��
��5��0��1 mol�ĵ���W��50 mL 1��5 mol��L��1��FeBr2��Һ��Ӧ����������Fe2����Br�������ʵ���֮����______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014���һ��ѧ�˽̰���� 4-2��Դ�ۺ����á�����������ϰ���������棩 ���ͣ������
�����ķ�����ʹ�ã��������ǻ۵Ľܳ����֣���Ϊ���ǵ���������������˼���ķ��㣬ͬʱ�ɴ������Ļ�����Ⱦ����ҲԽ��Խ�������ǵĹ�ע��
��1�������ŷŵ�β�������е������������Ⱦ��������ɲ����������������Ҫԭ���ǣ� ��
A��ȼ�պ���������ȼ�������
B��ȼ�պ�Ǧ���������
C������������ȼ�ղ���������
D���������е�N2���������ɵ�
��2�������йس��и�����ȾԴ������ɵĶ����Ӧ��ϵ��ȷ���ǣ� ��
A������β�����⻯ѧ��������ҵ���������ꣻ��ҵ��ˮ������������
B������β�������ꣻ��ҵ��������������������ҵ��ˮ���⻯ѧ����
C������β������������������ҵ���������ꣻ��ҵ��ˮ���⻯ѧ����
D������β�����⻯ѧ��������ҵ��������������������ҵ��ˮ������
��3��һ��������һ����̼��������β������к����ʣ������ܻ����ط������·�Ӧ��2NO��g����2CO��g��??N2��g����2CO2��g������H<0�������ô˷�Ӧ�������һ�ֻ���װ������������β���Դ�������Ⱦ��������Ʒ����������β������Ч������________��
��ѡ���ʵ��Ĵ����������װ�õ��¶ȡ�������װ�õ�ѹǿ������װ����װ���ʯ��
A���٢� B���ڢ�
C���٢� D���ڢ�
��4�����ͻ����������LPG�����͵�˫ȼ��ϵͳ����β���е��ж�����ɷֽ���ͨ�����½�80%���ң�����������β���ŷŸ����л�����ɵ���Ⱦ���⡣���������в����������к��������________��
A��CO2��H2 B��NO2��NO
C��CO��SO2 D��C���ͺ�Ǧ������
��5����ν����ɫ��������ָʹ��Һ��ʯ����������Ⱦ����Ⱦ��С����Դ��ȼ�ϵ���������ɫ�����ɱ����ж���Ǧ������ͬϵ���Լ������������ŷš�ȼ�յ�������Һ��ʯ��������Ҫ�ɷ��Ǻ�C3��C4��������������Ƚ�________��
A��ǰ�����ɵ�ˮ��
B��ǰ��������������
C��ǰ��ȼ�ղ�����������
D��ǰ�߲����ĺ�̼������������
��6��Ϊ�˼��ٴ�����Ⱦ����������ƹ�����ʹ�����ȼ�ϡ�Ŀǰʹ�õ����ȼ����Ҫ�����ࣺһ����ѹ����Ȼ������һ����Һ��ʯ������������ȼ�ϵ���Ҫ�ɷֶ���________��
A��̼ˮ������ B��̼�⻯����
C������ D������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014���һ��ѧ�˽̰���� 4-2��Դ�ۺ����á�����������ϰ���������棩 ���ͣ�ѡ����
���з�Ӧ��������ɫ��ѧ����ԭ����ǣ� ��
A�������᳧β����SO2��2NH3��H2O=��NH4��2SO3
B���������Ṥҵβ�������������Ⱦ��2NaOH��NO2��NO=2NaNO2��H2O
C���Ʊ�CuSO4��Cu��2H2SO4��Ũ��  CuSO4��SO2����2H2O
CuSO4��SO2����2H2O
D���Ʊ�CuSO4��2Cu��O2 2CuO��CuO��H2SO4��ϡ��=CuSO4��H2O
2CuO��CuO��H2SO4��ϡ��=CuSO4��H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014���һ��ѧ�˽̰���� 4-1-2��ˮ��Դ�Ŀ���������ϰ���������棩 ���ͣ�ѡ����
����Ԫ����Cl����Na����Br����I����Mg����U�����ں�ˮ�е���Ԫ�ص��ǣ� ��
A���٢ڢ� B���ܢ�
C���٢ڢۢ� D���ۢܢݢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014���һ��ѧ�˽̰���� 4-1-2��ˮ��Դ�Ŀ���������ϰ���������棩 ���ͣ�ѡ����
���дӺ�ˮ����ȡþ��ȷ�ķ����ǣ� ��
����ʾ���۵㣺MgO��2 850����MgCl2��714����
A����ˮ Mg��OH��2
Mg��OH��2 Mg
Mg
B����ˮ MgCl2��Һ��MgCl2����
MgCl2��Һ��MgCl2���� Mg
Mg
C����ˮ Mg��OH��2
Mg��OH��2 MgO
MgO Mg
Mg
D����ˮ Mg��OH��2
Mg��OH��2 MgCl2��Һ��MgCl2����
MgCl2��Һ��MgCl2���� Mg
Mg
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014���һ��ѧ�˽̰���� 4-1-1��������Ŀ���������ϰ���������棩 ���ͣ�ѡ����
����˵���У�����ȷ���ǣ� ��
A��������ұ������������������ԭ��Ӧԭ������һ�������½��������仯�����л�ԭ����
B��ұ������ʱ���������һ��������Ϊ��ԭ��
C�������ɻ���̬��Ϊ����̬�����DZ���ԭ
D���������ʱ����ֺ�Ӧ�õ�Խ�磬����һ��Խ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014���һ��ѧ�˽̰���� 3-3-2������ϰ���������棩 ���ͣ�ѡ����
������ӵĽṹʽΪ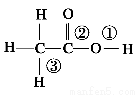 �����з�Ӧ���ϼ���λ��ȷ���ǣ� ��
�����з�Ӧ���ϼ���λ��ȷ���ǣ� ��
��1������ĵ��룬����������
��2���������Ҵ�����������Ӧ������������
��3���ں��״���ʱ��Br2��CH3COOH�ķ�Ӧ��CH3COOH��Br2 CH2Br��COOH��HBr������������
CH2Br��COOH��HBr������������
��4���������������ķ�Ӧ��2CH3COOH�D�� 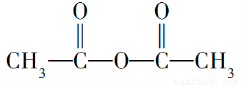 ��H2O�����٢�������
��H2O�����٢�������
A������1����2����3�� B����1����2����3����4��
C������2����3����4�� D������1����3����4��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com