
�����£���25 mL 0.1 mol/L MOH��Һ����μ���0.2 mol/L HA��Һ��������ͼ��ʾ������仯���Բ��ƣ����ش��������⣺
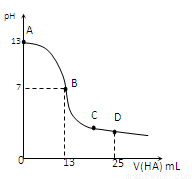
��1��д��MOH�ĵ��뷽��ʽ
��2��MOH��HAǡ����ȫ��Ӧʱ����Һ��_____�ԣ���ᡱ��������С����������ǣ������ӷ���ʽ��ʾ��__ _____��
��ʱ�������Һ����ˮ�������c(H+)__ _ 0.2 mol/L HA��Һ����ˮ�������c(H+)���������������=������
��3���ֱ�д��B��C���㣬�����Һ�и�����Ũ�ȵĴ�С��ϵ
B��_____________ _��C��___ __________��
��4��D��ʱ����Һ��c(A��)+c(HA)________2 c(M+)���������������=����������ʱ��û����Һ��pH = 3���� c(HA) + c(H+) = __________mol/L��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����£���25 mL 0.1 mol/L MOH��Һ����μ���0.2 mol/L HA��Һ��������ͼ��ʾ������仯���Բ��ƣ����ش��������⣺
��1��д��MOH�ĵ��뷽��ʽ
��2��MOH��HAǡ����ȫ��Ӧʱ����Һ��_____�ԣ���ᡱ��������С����������ǣ������ӷ���ʽ��ʾ��__ _____��
��ʱ�������Һ����ˮ�������c(H+)__ _ 0.2 mol/L HA��Һ����ˮ�������c(H+)���������������=������
��3���ֱ�д��B��C���㣬�����Һ�и�����Ũ�ȵĴ�С��ϵ
B��_____________ _��C��___ __________��
��4��D��ʱ����Һ��c(A��)+c(HA)________2 c(M+)���������������=����������ʱ��û����Һ��pH = 3���� c(HA) + c(H+) =__________mol/L��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�긣��ʡ���Ŵ�ѧ�����Ƽ���ѧ�߶���ѧ�����п���ѧ������������ ���ͣ������
�����£���25 mL 0.1 mol/L MOH��Һ����μ���0.2 mol/L HA��Һ��������ͼ��ʾ������仯���Բ��ƣ����ش��������⣺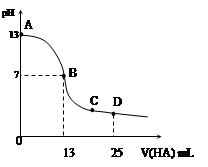
��1��д��MOH��ˮ��Һ�еĵ��뷽��ʽ
��2��MOH��HAǡ����ȫ��Ӧʱ����Һ��_____�ԣ���ᡱ��������С����������ǣ������ӷ���ʽ��ʾ��__ _____����ʱ�������Һ����ˮ�������c(H+)__ _ 0.2 mol/L HA��Һ����ˮ�������c(H+)���������������=������
��3��д��B������Һ�и�����Ũ�ȵĴ�С��ϵ_________ _��
��4��D��ʱ����Һ��c(A��)+c(HA)________2 c(M+)���������������=����������ʱ��û����Һ��pH = 3���� c(HA) + c(H+) = __________mol/L�����ֱ���ʽ�����ؾ�����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�츣��ʡ�߶���ѧ�����п���ѧ���������棩 ���ͣ������
�����£���25 mL 0.1 mol/L MOH��Һ����μ���0.2 mol/L HA��Һ��������ͼ��ʾ������仯���Բ��ƣ����ش��������⣺

��1��д��MOH��ˮ��Һ�еĵ��뷽��ʽ
��2��MOH��HAǡ����ȫ��Ӧʱ����Һ��_____�ԣ���ᡱ��������С����������ǣ������ӷ���ʽ��ʾ��__ _____����ʱ�������Һ����ˮ�������c(H+)__ _ 0.2 mol/L HA��Һ����ˮ�������c(H+)���������������=������
��3��д��B������Һ�и�����Ũ�ȵĴ�С��ϵ_________ _��
��4��D��ʱ����Һ��c(A��)+c(HA)________2 c(M+)���������������=����������ʱ��û����Һ��pH = 3���� c(HA) + c(H+) = __________mol/L�����ֱ���ʽ�����ؾ�����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�츣��ʡ�����и߶���ѧ����ĩ������ѧ�Ծ��������棩 ���ͣ������
�����£���25 mL 0.1 mol/L MOH��Һ����μ���0.2 mol/L HA��Һ��������ͼ��ʾ������仯���Բ��ƣ����ش��������⣺

��1��д��MOH��ˮ��Һ�еĵ��뷽��ʽ
��2��MOH��HAǡ����ȫ��Ӧʱ����Һ��_____�ԣ���ᡱ��������С����������ǣ������ӷ���ʽ��ʾ��__ _____����ʱ�������Һ����ˮ�������c(H+)__ _ 0.2 mol/L HA��Һ����ˮ�������c(H+)���������������=������
��3��д��B������Һ�и�����Ũ�ȵĴ�С��ϵ_________ _��
��4��D��ʱ����Һ��c(A��)+c(HA)________2 c(M+)���������������=����������ʱ��û����Һ��pH = 3���� c(HA) + c(H+) = __________mol/L�����ֱ���ʽ�����ؾ�����������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com