
| ׶ŠĪĘæÖŠµÄČÜŅŗ | µĪ¶Ø¹ÜÖŠµÄČÜŅŗ | Ń”ÓĆÖøŹ¾¼Į | Ń”ÓƵĪ¶Ø¹Ü | |
| A | ¼ī | Ėį | ŹÆČļ | ŅŅ |
| B | Ėį | ¼ī | ¼×»ł³Č | ¼× |
| C | ¼ī | Ėį | ·ÓĢŖ | ¼× |
| D | Ėį | ¼ī | ·ÓĢŖ | ŅŅ |



 Ģį·Ö°Ł·Ö°Ł¼ģ²ā¾ķĻµĮŠ“š°ø
Ģį·Ö°Ł·Ö°Ł¼ģ²ā¾ķĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĪŹ“šĢā
| µĪ¶Ø“ĪŹż | “ż²āĒāŃõ»ÆÄĘČÜŅŗµÄĢå»ż/mL | 0.1000mol/LŃĪĖįµÄĢå»ż£ØmL£© | ||
| µĪ¶ØĒ°æĢ¶Č | µĪ¶ØŗóæĢ¶Č | ČÜŅŗĢå/mL | ||
| µŚŅ»“Ī | 25.00 | 0.00 | 26.11 | 26.11 |
| µŚ¶ž“Ī | 25.00 | 1.56 | 30.30 | 28.74 |
| µŚČż“Ī | 25.00 | 0.22 | 26.31 | 26.09 |

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĪŹ“šĢā
| µĪ¶Ø“ĪŹż | “ż²ā²ŻĖįČÜŅŗĢå»ż£ØmL£© | 0.1000mol/LKMnO4±ź×¼ČÜŅŗĢå»ż£ØmL£© | |
| µĪ¶ØĒ°æĢ¶Č | µĪ¶ØŗóæĢ¶Č | ||
| µŚŅ»“Ī | 25.00 | 0.00 | 10.02 |
| µŚ¶ž“Ī | 25.00 | 0.22 | 11.32 |
| µŚČż“Ī | 25.00 | 1.56 | 11.54 |

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®²Ī¼Ó·“Ó¦µÄĖįŗĶ¼īµÄĪļÖŹµÄĮæĻąµČ |
| B£®µĪ¶ØŹ±£¬Ó¦Ź±æĢ×¢ŹÓµĪ¶Ø¹ÜÖŠŅŗĆęµÄ±ä»Æ |
| C£®pH=7Ź±£¬²Ī¼Ó·“Ó¦µÄĖįÖŠµÄH+×ÜĮæŗĶ¼īÖŠOH-×ÜĮæĻąµČ |
| D£®ĖįŗĶ¼īĒ”ŗĆĶźČ«·“Ó¦Ź±£¬·“Ó¦µÄČČŠ§Ó¦¼“ĪŖÖŠŗĶČČ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĪŹ“šĢā
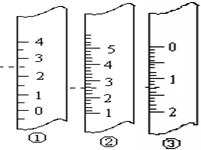
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
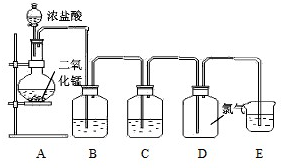
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĪŹ“šĢā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com