
| ||
| ||
| ||
| ||



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
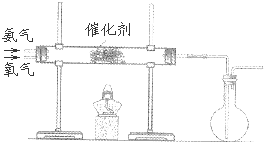
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
| ”÷ |
| µē½ā |
| 2800”ę |
| ”÷ |
| C |
| »¹Ō |
| HCl |
| ||
| 714”ę |
| HCl |
| »īĘĆ½šŹō |
| »¹Ō |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
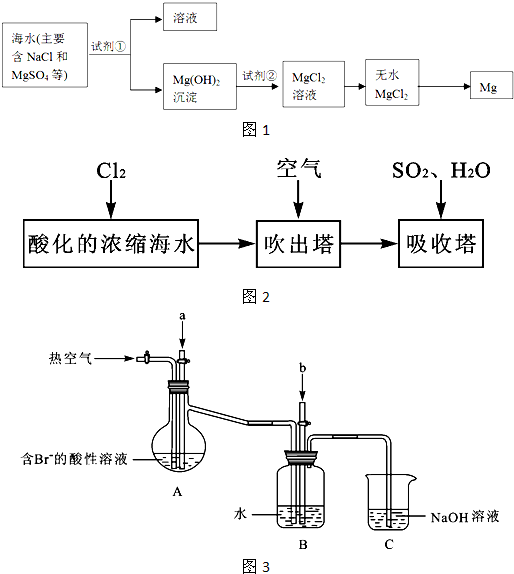
ŗ£ŃóŌ¼Õ¼µŲĒņ±ķĆ껿µÄ71%£¬ŗ£Ė®»Æѧ׏Ō“µÄĄūÓĆ¾ßÓŠ·Ē³£¹ćĄ«µÄĒ°¾°”£
£Ø1£©ŗ£Ė®É¹ŃĪæÉ»ńµĆ“ÖŃĪ£¬ŌŚŹµŃéŹŅÖŠ“ÖŃĪ¾¹żČܽā”¢ ”¢ æÉÖĘµĆ¾«ŃĪ”£
£Ø2£©Ć¾¼°ĘäŗĻ½šŹĒŅ»ÖÖÓĆĶ¾ŗܹćµÄ½šŹō²ÄĮĻ£¬ÄæĒ°ŹĄ½ēÉĻ60%µÄĆ¾ŹĒ“Óŗ£Ė®ÖŠĢįČ”µÄ£¬ĘäÖ÷ŅŖ²½ÖčČēĻĀ£ŗ
¢ŁĪŖĮĖŹ¹MgSO4×Ŗ»ÆĪŖMg(OH) 2£¬ŹŌ¼Į¢ŁæÉŅŌŃ”ÓĆ £¬ŅŖŹ¹MgSO4ĶźČ«×Ŗ»ÆĪŖ³Įµķ£¬¼ÓČėŹŌ¼Į¢ŁµÄĮæÓ¦ £»
¢ŚŹŌ¼Į¢ŚæÉŅŌŃ”ÓĆ £»
¢ŪŹŌ“Ó½ŚŌ¼ÄÜŌ“”¢Ģįøß½šŹōĆ¾µÄ“æ¶Č·ÖĪö£¬ŅŌĻĀŹŹŅĖµÄŅ±Ć¾·½·ØŹĒ ”£
A£® B£®
C£® D£®
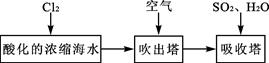
£Ø3£©äå¼°Ęä»ÆŗĻĪļÓĆĶ¾Ź®·Ö¹ć·ŗ£¬ĪŅ¹śÕżŌŚ“óĮ¦æŖÕ¹ŗ£Ė®ĢįäåµÄŃŠ¾æŗĶæŖ·¢¹¤×÷”£¹¤ŅµŅŌÅØĖõŗ£Ė®ĪŖŌĮĻĢįČ”äåµÄ²æ·Ö¹ż³ĢČēĻĀ£ŗ
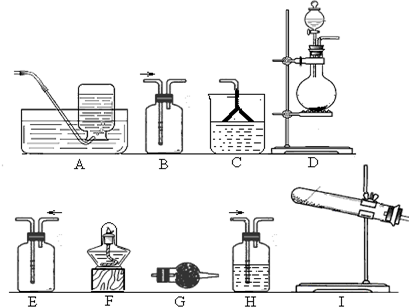
ijæĪĶāŠ”×éŌŚŹµŃéŹŅÄ£ÄāÉĻŹö¹ż³ĢÉč¼ĘŅŌĻĀ×°ÖĆ½ųŠŠŹµŃé£ØĖłÓŠĻš½ŗÖĘĘ·¾łŅѱ»±£»¤£¬¼Š³Ö×°ÖĆŅŃĀŌČ„£©£ŗ
¢ŁA×°ÖĆÖŠĶØČėaĘųĢåµÄÄæµÄŹĒ£ØÓĆĄė×Ó·½³ĢŹ½±ķŹ¾£© £»
¢ŚA×°ÖĆÖŠĶØČėaĘųĢåŅ»¶ĪŹ±¼äŗó£¬Ķ£Ö¹ĶØČė£¬øÄĶØČČæÕĘų”£ĶØČėČČæÕĘųµÄÄæµÄŹĒ
£»
¢Ū·“Ó¦¹ż³ĢÖŠ£¬B×°ÖĆÖŠÓŠSO42-Éś³É”£¼ģŃéSO42-µÄ·½·ØŹĒ £»
¢ÜC×°ÖƵÄ×÷ÓĆŹĒ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2010Äź¼ŖĮÖŹ”±±Ź¦“óÄž½ø½ÖŠøßŅ»ĻĀŃ§ĘŚĘŚÄ©æ¼ŹŌ»ÆѧŹŌĢā ĢāŠĶ£ŗĢīæÕĢā
ŗ£ŃóŌ¼Õ¼µŲĒņ±ķĆ껿µÄ71%£¬ŗ£Ė®»Æѧ׏Ō“µÄĄūÓĆ¾ßÓŠ·Ē³£¹ćĄ«µÄĒ°¾°”£
£Ø1£©ŗ£Ė®É¹ŃĪæÉ»ńµĆ“ÖŃĪ£¬ŌŚŹµŃéŹŅÖŠ“ÖŃĪ¾¹żČܽā”¢ ”¢ æÉÖĘµĆ¾«ŃĪ”£
£Ø2£©Ć¾¼°ĘäŗĻ½šŹĒŅ»ÖÖÓĆĶ¾ŗܹćµÄ½šŹō²ÄĮĻ£¬ÄæĒ°ŹĄ½ēÉĻ60%µÄĆ¾ŹĒ“Óŗ£Ė®ÖŠĢįČ”µÄ£¬ĘäÖ÷ŅŖ²½ÖčČēĻĀ£ŗ
¢ŁĪŖĮĖŹ¹MgSO4×Ŗ»ÆĪŖMg(OH) 2£¬ŹŌ¼Į¢ŁæÉŅŌŃ”ÓĆ £¬ŅŖŹ¹MgSO4ĶźČ«×Ŗ»ÆĪŖ³Įµķ£¬¼ÓČėŹŌ¼Į¢ŁµÄĮæÓ¦ £»
¢ŚŹŌ¼Į¢ŚæÉŅŌŃ”ÓĆ £»
¢ŪŹŌ“Ó½ŚŌ¼ÄÜŌ“”¢Ģįøß½šŹōĆ¾µÄ“æ¶Č·ÖĪö£¬ŅŌĻĀŹŹŅĖµÄŅ±Ć¾·½·ØŹĒ ”£
A£® | B£®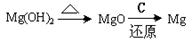 |
C£® | D£® |

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2010Äź±±¾©ŹŠ°ĖŅ»ÖŠŃ§øßŅ»µŚ¶žŃ§ĘŚĘŚÄ©æ¼ŹŌ»ÆѧŹŌ¾ķ ĢāŠĶ£ŗŹµŃéĢā
ŗ£ŃóŌ¼Õ¼µŲĒņ±ķĆ껿µÄ71%£¬ŗ£Ė®»Æѧ׏Ō“µÄĄūÓĆ¾ßÓŠ·Ē³£¹ćĄ«µÄĒ°¾°”£
£Ø1£©ŗ£Ė®É¹ŃĪæÉ»ńµĆ“ÖŃĪ£¬ŌŚŹµŃéŹŅÖŠ“ÖŃĪ¾¹żČܽā”¢ ”¢ æÉÖĘµĆ¾«ŃĪ”£
£Ø2£©Ć¾¼°ĘäŗĻ½šŹĒŅ»ÖÖÓĆĶ¾ŗܹćµÄ½šŹō²ÄĮĻ£¬ÄæĒ°ŹĄ½ēÉĻ60%µÄĆ¾ŹĒ“Óŗ£Ė®ÖŠĢįČ”µÄ£¬ĘäÖ÷ŅŖ²½ÖčČēĻĀ£ŗ
¢ŁĪŖĮĖŹ¹MgSO4×Ŗ»ÆĪŖMg(OH) 2£¬ŹŌ¼Į¢ŁæÉŅŌŃ”ÓĆ £¬ŅŖŹ¹MgSO4ĶźČ«×Ŗ»ÆĪŖ³Įµķ£¬¼ÓČėŹŌ¼Į¢ŁµÄĮæÓ¦ £»
¢ŚŹŌ¼Į¢ŚæÉŅŌŃ”ÓĆ £»
¢ŪŹŌ“Ó½ŚŌ¼ÄÜŌ“”¢Ģįøß½šŹōĆ¾µÄ“æ¶Č·ÖĪö£¬ŅŌĻĀŹŹŅĖµÄŅ±Ć¾·½·ØŹĒ ”£
£Ø3£©äå¼°Ęä»ÆŗĻĪļÓĆĶ¾Ź®·Ö¹ć·ŗ£¬ĪŅ¹śÕżŌŚ“óĮ¦æŖÕ¹ŗ£Ė®ĢįäåµÄŃŠ¾æŗĶæŖ·¢¹¤×÷”£¹¤ŅµŅŌÅØĖõŗ£Ė®ĪŖŌĮĻĢįČ”äåµÄ²æ·Ö¹ż³ĢČēĻĀ£ŗ
ijæĪĶāŠ”×éŌŚŹµŃéŹŅÄ£ÄāÉĻŹö¹ż³ĢÉč¼ĘŅŌĻĀ×°ÖĆ½ųŠŠŹµŃé£ØĖłÓŠĻš½ŗÖĘĘ·¾łŅѱ»±£»¤£¬¼Š³Ö×°ÖĆŅŃĀŌČ„£©£ŗ
¢ŁA×°ÖĆÖŠĶØČėaĘųĢåµÄÄæµÄŹĒ£ØÓĆĄė×Ó·½³ĢŹ½±ķŹ¾£© £»
¢ŚA×°ÖĆÖŠĶØČėaĘųĢåŅ»¶ĪŹ±¼äŗó£¬Ķ£Ö¹ĶØČė£¬øÄĶØČČæÕĘų”£ĶØČėČČæÕĘųµÄÄæµÄŹĒ
£»
¢Ū·“Ó¦¹ż³ĢÖŠ£¬B×°ÖĆÖŠÓŠSO42-Éś³É”£¼ģŃéSO42-µÄ·½·ØŹĒ £»
¢ÜC×°ÖƵÄ×÷ÓĆŹĒ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2010Äź¼ŖĮÖŹ”øßŅ»ĻĀŃ§ĘŚĘŚÄ©æ¼ŹŌ»ÆѧŹŌĢā ĢāŠĶ£ŗĢīæÕĢā
ŗ£ŃóŌ¼Õ¼µŲĒņ±ķĆ껿µÄ71%£¬ŗ£Ė®»Æѧ׏Ō“µÄĄūÓĆ¾ßÓŠ·Ē³£¹ćĄ«µÄĒ°¾°”£
£Ø1£©ŗ£Ė®É¹ŃĪæÉ»ńµĆ“ÖŃĪ£¬ŌŚŹµŃéŹŅÖŠ“ÖŃĪ¾¹żČܽā”¢ ”¢ æÉÖĘµĆ¾«ŃĪ”£
£Ø2£©Ć¾¼°ĘäŗĻ½šŹĒŅ»ÖÖÓĆĶ¾ŗܹćµÄ½šŹō²ÄĮĻ£¬ÄæĒ°ŹĄ½ēÉĻ60%µÄĆ¾ŹĒ“Óŗ£Ė®ÖŠĢįČ”µÄ£¬ĘäÖ÷ŅŖ²½ÖčČēĻĀ£ŗ

¢ŁĪŖĮĖŹ¹MgSO4×Ŗ»ÆĪŖMg(OH) 2£¬ŹŌ¼Į¢ŁæÉŅŌŃ”ÓĆ £¬ŅŖŹ¹MgSO4ĶźČ«×Ŗ»ÆĪŖ³Įµķ£¬¼ÓČėŹŌ¼Į¢ŁµÄĮæÓ¦ £»
¢ŚŹŌ¼Į¢ŚæÉŅŌŃ”ÓĆ £»
¢ŪŹŌ“Ó½ŚŌ¼ÄÜŌ“”¢Ģįøß½šŹōĆ¾µÄ“æ¶Č·ÖĪö£¬ŅŌĻĀŹŹŅĖµÄŅ±Ć¾·½·ØŹĒ ”£
A£® B£®
B£®
C£®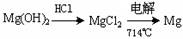 D£®
D£®
£Ø3£©äå¼°Ęä»ÆŗĻĪļÓĆĶ¾Ź®·Ö¹ć·ŗ£¬ĪŅ¹śÕżŌŚ“óĮ¦æŖÕ¹ŗ£Ė®ĢįäåµÄŃŠ¾æŗĶæŖ·¢¹¤×÷”£¹¤ŅµŅŌÅØĖõŗ£Ė®ĪŖŌĮĻĢįČ”äåµÄ²æ·Ö¹ż³ĢČēĻĀ£ŗ

ijæĪĶāŠ”×éŌŚŹµŃéŹŅÄ£ÄāÉĻŹö¹ż³ĢÉč¼ĘŅŌĻĀ×°ÖĆ½ųŠŠŹµŃé£ØĖłÓŠĻš½ŗÖĘĘ·¾łŅѱ»±£»¤£¬¼Š³Ö×°ÖĆŅŃĀŌČ„£©£ŗ
¢ŁA×°ÖĆÖŠĶØČėaĘųĢåµÄÄæµÄŹĒ£ØÓĆĄė×Ó·½³ĢŹ½±ķŹ¾£© £»
¢ŚA×°ÖĆÖŠĶØČėaĘųĢåŅ»¶ĪŹ±¼äŗó£¬Ķ£Ö¹ĶØČė£¬øÄĶØČČæÕĘų”£ĶØČėČČæÕĘųµÄÄæµÄŹĒ
£»
¢Ū·“Ó¦¹ż³ĢÖŠ£¬B×°ÖĆÖŠÓŠSO42-Éś³É”£¼ģŃéSO42-µÄ·½·ØŹĒ £»
¢ÜC×°ÖƵÄ×÷ÓĆŹĒ ”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com