
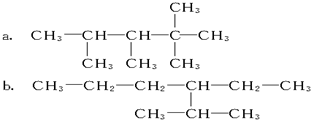
���� ��1����2����3���ж��л���������Ƿ���ȷ����л�������������������ȷ���������淶��
��������ԭ��
�ٳ���ѡ�̼��Ϊ������
�ڶࣺ���ȳ�̼��ʱ��֧�����Ϊ������
�۽�����֧�����һ�˱�ţ�
��С��֧�����֮����С��������ṹ��ʽ�����Ҷ˻���˿��������ϡ���-----��֧�����һ�˱�š���ԭ��
�ݼ���ȡ���������������˵Ⱦ���ʱ���Ӽ�ȡ������ʼ��ţ���ȡ������ͬ���ͰѼ�д��ǰ�棬���ӵ�д�ں��棻
�л����������дҪ�淶��
���ڽṹ�к��б����ģ�����ʱ�������α��������Ҳ���Ը��������λ�ã��á��ڡ������䡱�����ԡ�����������
���й����ŵ��л�������ʱ��Ҫѡ�������ŵ��̼����Ϊ�����������ŵ�λ����С��
��4��C5H11ClΪ�����һ�ȴ���жϺ���д������һ�ȴ�����칹����������²���������
��ȷ��������̼���칹����������ͬ���칹�壻�ҳ���Ч����ԭ�ӣ����������ĺ����ߵ�ԭ����ԭ����һȥ������ԭ�ӣ��ݴ��жϷ���������ͬ���칹����Ŀ��
��� �⣺��1��2��3��5-����-3��4-���һ����飬��̼����7��̼ԭ�ӣ�2��3��5��̼���м���3��4��̼�����һ���д���Ľṹ��ʽΪ��CH3CH��CH3��C��CH3����CH2CH3��CH��CH2CH3��CH��CH3��CH3��
�ʴ�Ϊ��CH3CH��CH3��C��CH3����CH2CH3��CH��CH2CH3��CH��CH3��CH3��
��2��2��3-����-4-�һ����飬��̼��6��̼ԭ�ӣ�2��3��̼���м���4��̼�����һ����ݴ���д�õ��ṹ��ʽΪ��CH3CH��CH3��CH��CH3��CH��CH2CH3��CH2CH3��
�ʴ�Ϊ��CH3CH��CH3��CH��CH3��CH��CH2CH3��CH2CH3��
��3��a��ѡȡ�̼��Ϊ��̼������5��̼����ȡ��������һ�˱��ȷ��ȡ����λ�ã�����Ϊ��2.2.3.4-�ļ����飬�ʴ�Ϊ��2.2.3.4-�ļ����飻
b��ѡȡ�̼��Ϊ��̼������6��̼��ȡ������࣬��ȡ��������һ�˱��ȷ��ȡ����λ�ã�����Ϊ��2-��-3-�һ����飬�ʴ�Ϊ��2-��-3-�һ����飻
��4�������ͬ���칹����CH3-CH2-CH2-CH2-CH3�� ��
�� ��
��
��ΪCH3-CH2-CH2-CH2-CH3��һ�ȴ����У�CH3CH2CH2CH2CH2Cl��CH3CH2CH2CHClCH3��CH3CH2CHClCH2CH3��
����CH3CH2CH2CHClCH3��CH3CH2CHClCH2CH3����������
��Ϊ ��һ�ȴ����У�CH3CH��CH3��CH2CH2Cl��CH3CH��CH3��CHClCH3��CH3CCl��CH3��CH2CH3��CH2ClCH��CH3��CH2CH3��
��һ�ȴ����У�CH3CH��CH3��CH2CH2Cl��CH3CH��CH3��CHClCH3��CH3CCl��CH3��CH2CH3��CH2ClCH��CH3��CH2CH3��
��Ϊ ��һ�ȴ��CH3C��CH3��2CH2Cl��
��һ�ȴ��CH3C��CH3��2CH2Cl��
�����в���֧���Ľṹ��ʽΪ��CH3CH2CH2CH2CH2Cl��CH3CH2CH2CHClCH3��CH3CH2CHClCH2CH3��
�ʴ�Ϊ��CH3CH2CH2CHClCH3��CH3CH2CHClCH2CH3��
���� ���⿼���������������ṹ��ʽ��д����������������ȴ�����������ͬ���칹����жϣ���Ŀ�ѶȲ���ע�����ճ����л��������ԭ��ͬ���칹����д��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ���Ӵ��� | a | I | e |
| ԭ�Ӻ��� | ���� | �ĺ� | ���� |
| ���ӵĵ���� | һ����λ����� | 0 | һ����λ����� |

 ����
���� ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 3NO2+H2�T2HNO3+NO | B�� | 4FeS2+11O2$\frac{\underline{\;\;��\;\;}}{\;}$2Fe2O3+8SO2 | ||
| C�� | 3Cl2+8NH3�TN2+6NH4Cl | D�� | 2Na+2NH3��Һ���T2NaNH2+H2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
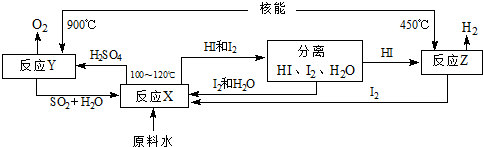
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
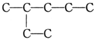
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
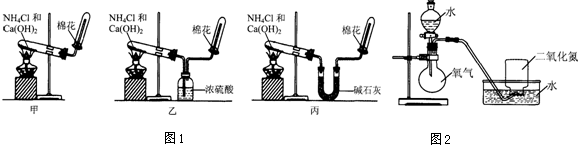
| ʵ����� | ʵ������ | |
| ����һ | ���ռ���NO2�ļ���ƿ������ˮ���У� | ����ƿ��Һ������������ƿ�������ɺ���ɫ�����ɫ������ʣ������Լռ����ƿ���������֮һ�� |
| ����� | ����Һ©���е�ˮ��ε�����ƿ��ʹ����O2����ʢ��NO2�ļ���ƿ��ֹͣ��ˮ�� | ����ƿ��������ɫ�ı仯�����������ɫ��Ϊ����ɫ���ٱ����ɫ������ƿ��Һ����������� |
| ������ | ����ظ�����������������μ���O2ͨ������ֱ��O2ͨ������岻�ٱ�ɺ���ɫ�� | �����벽�����ͬ�������ƿ�ڼ�������Һ�壬ֻ�����������壮 |
| ���� | ������ʵ�鷽��������״����448mLNO2ȫ������ˮ���õ�500mL��Һ������Һ��HNO3�����ʵ���Ũ��Ϊ0.04mol/L�� | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
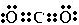 ������ת����������ԭ�ϣ������ƻ��Ļ�ѧ�����ڼ��ԣ�����ԡ��Ǽ��ԡ�������
������ת����������ԭ�ϣ������ƻ��Ļ�ѧ�����ڼ��ԣ�����ԡ��Ǽ��ԡ�������| ���ۼ� | H-H | H-F | H-Cl | H-Br | H-O | H-S | H-N | H-P |
| ������pm�� | 74 | 92 | 127 | 141 | 98 | 135 | 101 | 321 |
| ���ܣ�kJ/mol�� | 436 | 568 | 432 | 368 | 464 | 364 | 391 | 142 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ɷǽ���Ԫ����ɵĻ����ﲻһ�����ǹ��ۻ����� | |
| B�� | ���ۻ�������һ�����м��Լ� | |
| C�� | �������Ӽ��Ļ�����һ�������ӻ����� | |
| D�� | �������嵥�ʷ�����һ�����зǼ��Լ� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com