
ĻĀĶ¼ĪŖijŹŠŹŪŃĪĖįŹŌ¼ĮĘæ±źĒ©ÉĻµÄ²æ·ÖŹż¾Ż”£ĪŹ£ŗ
| ŃĪ Ėį ·Ö×ÓŹ½£ŗHCl Ļą¶Ō·Ö×ÓÖŹĮæ£ŗ36.5 Ķā¹ŪŗĻøń ĆܶČŌ¼£ŗ1.18 g/cm3 HClµÄÖŹĮæ·ÖŹż£ŗ36.5% ·ūŗĻGB622~89 ŹŌ¼ĮÉś²śŠķæÉÖ¤±ąŗÅ£ŗ |
£Ø1£©øĆŃĪĖįµÄĪļÖŹµÄĮæÅضČĪŖ¶ąÉŁ£æ£ØĮŠŹ½¼ĘĖć£©
£Ø2£©Č”øĆŃĪĖį25.5 mLÓė2.00 mol/LµÄĒāŃõ»ÆÄĘČÜŅŗ100 mL»ģŗĻ£¬ŌŁ½«»ģŗĻŗóČÜŅŗĻ”ŹĶÖĮ1.00 L£¬“ĖŹ±ČÜŅŗµÄpHŌ¼ĪŖ¶ąÉŁ£æ
½āĪö£ŗ£Ø1)c(HCl)=1000 mL”Į1.18 g/cm3”Į36.5%/36.5 g/mol”Į1 L=11.8 mol/L”£
£Ø2)n(HCl)=11.8 mol/L”Į0.0255 L”Ö0.300 mol,n(NaOH)=2.00 mol/L”Į0.100 L=0.200 mol,
»ģŗĻ²¢Ļ”ŹĶŗóČÜŅŗÖŠc(H+)=0.300 mol-0.200 mol/1.00 L=0.100 mol/L,pH=-lgc(H+)=1.
“š°ø£ŗ£Ø1)c(HCl)=1000 mL”Į1.18 g/cm3”Į36.5%/36.5 g/mol”Į1 L=11.8 mol/L
£Ø2)1


 ŠÄĖćæŚĖćĒÉĖćŅ»æĪŅ»Į·ĻµĮŠ“š°ø
ŠÄĖćæŚĖćĒÉĖćŅ»æĪŅ»Į·ĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2013½ģ½Ī÷Ź”ÉĻČÄĻŲ֊ѧøßČżµŚŅ»“ĪŌĀæ¼£ØĢŲ£©»ÆѧŹŌ¾ķ£Ø“ų½āĪö£© ĢāŠĶ£ŗĢīæÕĢā
£Ø12·Ö£©£Ø1£©ĻĀĶ¼ĪŖijŹŠŹŪŃĪĖįŹŌ¼ĮĘæ±źĒ©ÉĻµÄ²æ·ÖŹż¾Ż£®ĪŹ£ŗ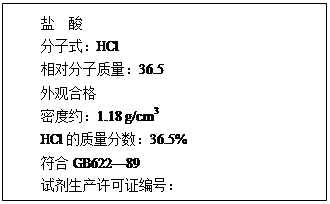
øĆŃĪĖįµÄĪļÖŹµÄĮæÅضČĪŖ¶ąÉŁ£æ(ĮŠŹ½¼ĘĖć)
(2) ŅŃÖŖŠæŗĶĀĮ¶¼ŹĒ»īĘĆ½šŹō£¬ĘäĒāŃõ»ÆĪļ¼ČÄÜČÜÓŚĒæĖį£¬ÓÖÄÜČÜÓŚĒæ¼ī”£µ«ŹĒĒāŃõ»ÆĀĮ²»ČÜÓŚ°±Ė®£¬¶ųĒāŃõ»ÆŠæÄÜČÜÓŚ°±Ė®£¬Éś³ÉZn£ØNH3£©42+”£Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©µ„ÖŹĀĮČÜÓŚĒāŃõ»ÆÄĘČÜŅŗŗó£¬ČÜŅŗÖŠĀĮŌŖĖŲµÄ“ęŌŚŠĪŹ½ĪŖ £ØÓĆ»ÆѧŹ½±ķŹ¾£©”£
£Ø2£©Š“³öŠæŗĶĒāŃõ»ÆÄĘČÜŅŗ·“Ó¦µÄ»Æѧ·½³ĢŹ½ ”£
£Ø3£©ĻĀĮŠø÷×éÖŠµÄĮ½ÖÖČÜŅŗ£¬ÓĆĻą»„µĪ¼ÓµÄŹµŃé·½·Ø¼“æɼų±šµÄŹĒ ”£
¢ŁĮņĖįĀĮŗĶĒāŃõ»ÆÄĘ ¢ŚĮņĖįĀĮŗĶ°±Ė®
¢ŪĮņĖįŠæŗĶĒāŃõ»ÆÄĘ ¢ÜĮņĖįŠæŗĶ°±Ė®
£Ø4£©Š“³öæÉČÜŠŌĀĮŃĪÓė°±Ė®·“Ó¦µÄĄė×Ó·½³ĢŹ½ .”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012-2013ѧğ½Ī÷Ź”øßČżµŚŅ»“ĪŌĀæ¼£ØĢŲ£©»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
£Ø12·Ö£©£Ø1£©ĻĀĶ¼ĪŖijŹŠŹŪŃĪĖįŹŌ¼ĮĘæ±źĒ©ÉĻµÄ²æ·ÖŹż¾Ż£®ĪŹ£ŗ
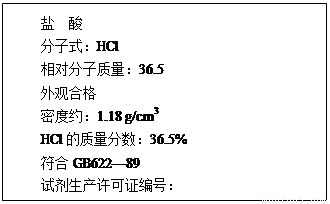
øĆŃĪĖįµÄĪļÖŹµÄĮæÅضČĪŖ¶ąÉŁ£æ(ĮŠŹ½¼ĘĖć)
(2) ŅŃÖŖŠæŗĶĀĮ¶¼ŹĒ»īĘĆ½šŹō£¬ĘäĒāŃõ»ÆĪļ¼ČÄÜČÜÓŚĒæĖį£¬ÓÖÄÜČÜÓŚĒæ¼ī”£µ«ŹĒĒāŃõ»ÆĀĮ²»ČÜÓŚ°±Ė®£¬¶ųĒāŃõ»ÆŠæÄÜČÜÓŚ°±Ė®£¬Éś³ÉZn£ØNH3£©42+”£Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©µ„ÖŹĀĮČÜÓŚĒāŃõ»ÆÄĘČÜŅŗŗó£¬ČÜŅŗÖŠĀĮŌŖĖŲµÄ“ęŌŚŠĪŹ½ĪŖ £ØÓĆ»ÆѧŹ½±ķŹ¾£©”£
£Ø2£©Š“³öŠæŗĶĒāŃõ»ÆÄĘČÜŅŗ·“Ó¦µÄ»Æѧ·½³ĢŹ½ ”£
£Ø3£©ĻĀĮŠø÷×éÖŠµÄĮ½ÖÖČÜŅŗ£¬ÓĆĻą»„µĪ¼ÓµÄŹµŃé·½·Ø¼“æɼų±šµÄŹĒ ”£
¢ŁĮņĖįĀĮŗĶĒāŃõ»ÆÄĘ ¢ŚĮņĖįĀĮŗĶ°±Ė®
¢ŪĮņĖįŠæŗĶĒāŃõ»ÆÄĘ ¢ÜĮņĖįŠæŗĶ°±Ė®
£Ø4£©Š“³öæÉČÜŠŌĀĮŃĪÓė°±Ė®·“Ó¦µÄĄė×Ó·½³ĢŹ½ .”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011-2012ѧğÕć½Ź”øßČżÉĻŃ§ĘŚĘŚÖŠæ¼ŹŌ»ÆѧŹŌ¾ķ ĢāŠĶ£ŗĢīæÕĢā
£Ø5·Ö£©ĻĀĶ¼ĪŖijŹŠŹŪŃĪĖįŹŌ¼ĮĘæ±źĒ©ÉĻµÄ²æ·ÖŹż¾Ż”£ĪŹ£ŗ

£Ø1£©øĆŃĪĖįµÄĪļÖŹµÄĮæÅضČĪŖ¶ąÉŁ£æ
£Ø2£©Č”øĆŃĪĖį25.4 mLÓė2.00 mol”¤L-1µÄĒāŃõ»ÆÄĘ
ČÜŅŗ100 mL»ģŗĻ£¬ŌŁ½«»ģŗĻŅŗĻ”ŹĶµ½1.00 L£¬
“ĖŹ±ČÜŅŗµÄpHŌ¼ĪŖ¶ąÉŁ£æ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŃĪ Ėį ·Ö×ÓŹ½£ŗHCl Ļą¶Ō·Ö×ÓÖŹĮæ£ŗ36.5 Ķā¹ŪŗĻøń ĆܶČŌ¼£ŗ HClµÄÖŹĮæ·ÖŹż£ŗ36.5% ·ūŗĻGB622-89 ŹŌ¼ĮÉś²śŠķæÉÖ¤±ąŗÅ£ŗ |
(1)øĆŃĪĖįµÄĪļÖŹµÄĮæÅضČĪŖ¶ąÉŁ£æ(ĮŠŹ½¼ĘĖć)
(2)Č”øĆŃĪĖį25.4 mLÓė2.00 mol”¤L-1µÄĒāŃõ»ÆÄĘČÜŅŗ100 mL»ģŗĻ£¬ŌŁ½«»ģŗĻŗóČÜŅŗĻ”ŹĶÖĮ
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com