



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
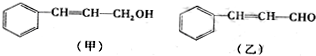 ��
��| ����ʽ | C16H14O2 |
| �������� | ��ʹBr2/CCl4��ɫ |
| ����ϡH2SO4��ˮ�� |
| һ������ |
| �� |
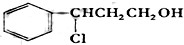
| O2/Cu |
| �� |
| һ������ |
| �� |
| ��O3 |
| ��Zn/H2O |
| ��ŨNaOH |
| ��H+ |
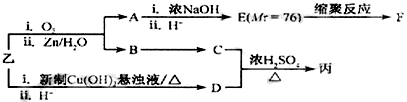
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��Mg2+��H+��SO42-��Cl- |
| B��Ca2+��H+��NO3-��CO32- |
| C��Fe3+��Na+��SCN-��OH- |
| D��Cu2+��NH4+��Cl-��OH- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���ƵĻ�ԭ�� |
| B��������ȼ��ʱ������ɫ���� |
| C���ƹ����Զ������ǿ |
| D�����۳�С����ˮ���Ĵ��ζ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| �� | �� | �� | �� | |
| pH | 11 | 11 | 3 | 3 |
| ��Һ | ��ˮ | ����������Һ | ���� | ���� |
| A���١����зֱ�����������Ȼ�茶��������Һ��pH����С |
| B���ֱ��ˮϡ��10����������Һ��pH���٣��ڣ��ܣ��� |
| C���١�������Һ�������ϣ�������Һ��c��Cl-����c��NH4+����c��OH-����c��H+�� |
| D��VaL����VbL����Һ��Ϻ�����Ϻ���ҺpH=7����Va��Vb=1��11 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A�����淴Ӧ��������� |
| B�����淴Ӧ�����ڼ������� |
| C�����淴Ӧ�����ʾ�Ϊ�� |
| D����Ӧ������и���ֵ�Ũ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���۵㣺CO2��KCl��SiO2 |
| B���������������Cl-��CH3COO-��OH- |
| C���е㣺���飾���飾���� |
| D�����ȶ��ԣ�HF��H2O��NH3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��78gNa2O2�����к��е���������ΪNA�����ԭ������ Na��23 O��16 H��1�� |
| B����״���£�11.2L��ϩ���еĹ��ۼ���ĿΪ3 NA |
| C����0�棬101ǧ��ʱ�������ƴ�ˮ���û���22.4LH2������ת�Ƶĵ�����Ϊ2NA |
| D����25�棬pH=13��NaOH��Һ�к��е�OH-��ԼΪ6.02��1022 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com