
| |||||||||||||||
 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

| BL |
| 22.4L/mol |
| Ag |
| 100g/mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011�콭��ʡ�Ž��и���2����У���������ۣ���ѧ���� ���ͣ�ʵ����
������ˮ�к��ж�������,ijУ��ѧ�о���ѧϰС���ͬѧΪ̽��������,��������ʵ��,���������ɣ�
��1��������ʹʪ��ĺ�ɫ������ɫ��ʹ����ɫ�����Ļ�ѧʽ��______
��2������ˮ�ڹ�����һ��ʱ�䣬��Һ��ɫ��dz�����йط�Ӧ�Ļ�ѧ����ʽΪ��
��
��3��ƽ�ⳣ�������˷����ϵ�Ŀ��淴Ӧ�ڸ������¶��½��еij̶ȣ�����ͬһ�����͵ķ�Ӧ��ƽ�ⳣ��Խ������Ӧ���еij̶� Խ��
Խ��
H2CO3 
 �� H�� Ka1��H2CO3��=4.45��10��7
�� H�� Ka1��H2CO3��=4.45��10��7

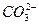 ��H�� Ka2(HCO3��)=5.61��10��11
��H�� Ka2(HCO3��)=5.61��10��11
HclO  H����
H���� Ka(HClO)=2.95��10��8
Ka(HClO)=2.95��10��8
���������ϵ���ƽ�ⳣ������д��������������ͨ�뵽������̼������Һ����������Ӧ�����ӷ���ʽ����
��4��������ˮ��ʯ��ʯ�ķ�Ӧ����ȡ��ŨHClO��Һ�ķ���֮һ��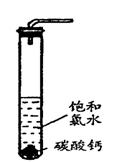
ʵ��һ�������о���
�� ���Թ��м�������Ŀ�״̼��ƣ��ټ���Լ20mL������ˮ����ַ�Ӧ��
���������ݲ�������Һdz����ɫ��ȥ��
�� ���ˣ�����Һ������ɫ�����ϣ����������ˮ��Ư���Ը�ǿ��
�� Ϊ��ȷ����Ӧ�������Һ��Ϊ���ݣ��ֱ��������ʵ�飺
��һ����ʯ��ˮ��ϣ���������������ɫ������
�ڶ�����ϡ�����ϣ���������������ɫ���壻
�������ݼ��ȣ�������Һ��������д�����ɫ���������
����⣬����ʵ���в�������ɫ�����ΪCO2��
��ش�
�� ��Ӧ�����õ���ҺƯ������ǿ��ԭ����______ ___________ ____
����������ʵ�����֪���ڵ���Һ�е����ʳ�CaCl2��HClO�⣬������_______ ��
ʵ����������о���
��Բ����ƿ�ײ�����һ����������ס�Ĺ�����״ ̼��ƺ�150mL������ˮ������ͼ��ʾװ��ʵ�飬�����ٲ������ݺ���������ʣ���ʯ��ʯ���Һ �棬�ܷ���ټ��ȡ������ƿ�е�Һ�壬ֱ����Ͳ�е����岻�����ӣ�������HClO�ķֽ⣩��
�棬�ܷ���ټ��ȡ������ƿ�е�Һ�壬ֱ����Ͳ�е����岻�����ӣ�������HClO�ķֽ⣩��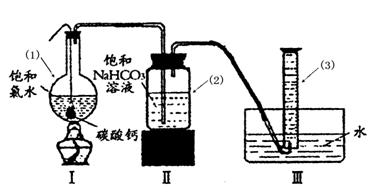
��ش�
���������1����������3��������
��1�� ��3��
��Ϊ������װ�â��ռ����������CO2���ܽ����ɵ���ʧ����ˮ������ȻΪˮ�������װ�â���иĽ�����ķ����� ��
�����ȷ������Ͳ����������
a________ _
b �����ƶ���Ͳ����Ͳ��Һ����ˮ��Һ����ƽ
c
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ڳ����£�Fe��ˮ������Ӧ�����ڸ����£�Fe��ˮ�����ɷ�����Ӧ��
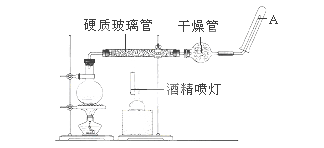
Ӧ������װ�ã���Ӳ�ʲ������з��뻹ԭ���ۺ�ʯ���Ļ������ȣ���ͨ��ˮ�������Ϳ�����ɸ����¡�Fe��ˮ�����ķ�Ӧʵ�顱��
��ش��ʵ���е����⡣
��1��д���÷�Ӧ�ķ�Ӧ����ʽ���������������������������� ����������������ָ����������ԭ��Ӧ�Ļ�ԭ���������������� �������������������� ��
��2��ʵ��ǰ���������װ�ý��������Լ�飬�������������������������� ��
��3��Բ����ƿ��ʢװ��ˮ����װ�����Ⱥ����Ҫ���������������������� ����ƿ�ײ������˼�Ƭ���Ƭ�����Ƭ������������������������������ ��
��4���������ʢװ�ǵ������������������� ���������������������������� ��
�� 5���Թ����ռ����������������� �����Ҫ��A�������ܴ���ȼ�����壬�����Ը�������������������������� �������������������������� ����һ������Ŀ������������������������������������������������ ��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ڳ����£�Fe��ˮ������Ӧ�����ڸ����£�Fe��ˮ�����ɷ�����Ӧ��
Ӧ������װ�ã���Ӳ�ʲ������з��뻹ԭ���ۺ�ʯ���Ļ������ȣ���ͨ��ˮ�������Ϳ�����ɸ����¡�Fe��ˮ�����ķ�Ӧʵ�顱��
��ش��ʵ���е����⡣
��1��д���÷�Ӧ�ķ�Ӧ����ʽ���������������������������������������� ��
��2��ʵ��ǰ������еIJ����������������������� ��
��3��Բ����ƿ��ʢװ��ˮ����װ�����Ⱥ����Ҫ�������������������������� ����ƿ�ײ������˼�Ƭ���Ƭ�����Ƭ���������������������������������������� ��
��4���ƾ��ƺ;ƾ���Ƶ�ȼ��˳���������������������� �������������������� ��
��5���������ʢװ�ǵ������������������������� ���������������������������� ��
��6���Թ����ռ����������������� �����Ҫ��A�������ܴ���ȼ�����壬�����Ը�������������������� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com