
| A�� | �ð�ˮ����������SO2���壺2OH-+SO2�TSO32-+H2O | |
| B�� | NaAlO2��Һ��AlO2-��ˮ�⣺AlO2-+2H2O�TAl��OH��3��+OH- | |
| C�� | ����NaClO����ˮ�е�NH3������N2��3ClO-+2NH3�TN2��+3Cl-+3H2O | |
| D�� | NaHCO3��Һ�м�����Ba��OH��2��Һ��HCO3-+Ba2++OH-�TBaCO3��+H2O |
���� A������������������Ӧ������������泥�
B��ƫ���������ˮ��̶Ƚ�С�����ɵ�������������ʹ�ó������ţ�
C���������ƾ���ǿ�����ԣ��ܹ������������ɵ�����
D������������������Ӧ����̼�ᱵ��̼���ƺ�ˮ��
��� �⣺A���ð�ˮ����������SO2���壬��Ӧ������������泥���ȷ�����ӷ���ʽΪ��OH-+SO2�THSO3-+H2O����A����
B��NaAlO2��Һ��AlO2-��ˮ�������������������������ӣ���ȷ�����ӷ���ʽΪ��AlO2-+2H2O�TAl��OH��3+OH-����B����
C��NaClO����ˮ�е�NH3������N2���Ȼ��ƺ�ˮ����Ӧ�����ӷ���ʽΪ��3ClO-+2NH3�TN2��+3Cl-+3H2O����C��ȷ��
D��̼��������Һ�м�����Ba��OH��2��Һ����Ӧ����̼�ᱵ������̼���ƺ�ˮ����ȷ�����ӷ���ʽΪ��2HCO3-+Ba2++2OH-�TBaCO3��+2H2O+CO32-����D����
��ѡC��
���� ���⿼�������ӷ���ʽ����д�жϣ�Ϊ�߿��ĸ�Ƶ�⣬��Ŀ�Ѷ��еȣ�ע����ȷ���ӷ���ʽ�����жϳ��÷�������鷴Ӧ�ܷ�������鷴Ӧ��������Ƿ���ȷ���������ʲ���Ƿ���ȷ���������������ʵ���Ҫ������ѧʽ������Ƿ����ԭ��ѧ����ʽ�ȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ʵ��Ŀ�� | ��� |
| �����Ȼ������Ƿ���� | |
| ��ȥʳ��������ϸɰ | |
| ��ȥ̼���ƹ���������̼������ | |
| ��ȥþ���л��е��������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
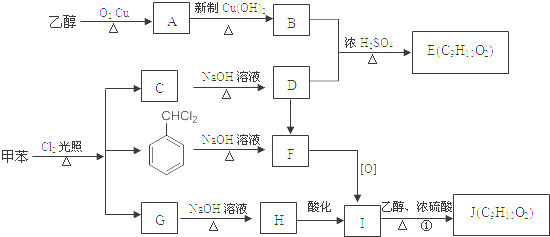

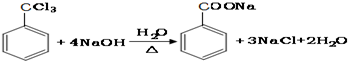 ��
��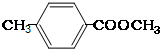 ����
����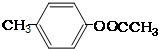 ����
����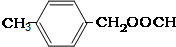 ��
��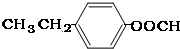 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | P�������� | B�� | ������H2O2��Na2H2P2O6 | ||
| C�� | 1mol H2O2��Ӧ��ת�Ƶ���1mol | D�� | Na2H2P2O6����Ԫ�صĻ��ϼ�Ϊ+3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��Fe��NO3��2ϡ��Һ�м������3Fe2++4H++NO3-�T3Fe3++NO��+2H2O | |
| B�� | ͭƬ��ŨHNO3��Cu+2NO3-+4H+�TCu2++NO2��+2H2O | |
| C�� | �Ȼ��Ũ��Һ��ŨNaOH��Һ��Ϻ���ȣ�NH4++OH-$\frac{\underline{\;\;��\;\;}}{\;}$NH3��+H2O | |
| D�� | ̼�������Һ��������NaOH��Һ��Ϻ���ȣ�NH4++OH-$\frac{\underline{\;\;��\;\;}}{\;}$NH3��+H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Ϊ�ⶨ������ˮ��pH���ò�����պȡҺ�����pH��ֽ�ϣ������ɫ�����ռ��� | |
| B�� | ����պ��Ũ������ް��������Ͱ����Ĺܵ��Ƿ�©�� | |
| C�� | ��������������Ԫ�أ�����Ҫ��Ը����ߵ����ʳ�� | |
| D�� | �ᴿ������������ص��Ȼ��ƣ�Ӧ�����ڽϸ��¶����Ƶ�Ũ��Һ����ȴ�ᾧ�����ˡ�����ķ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
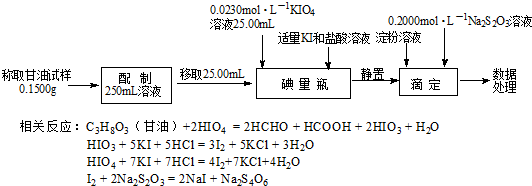
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ۢ� | B�� | �٢� | C�� | ���Т� | D�� | �ڢ� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com