
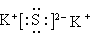 ��2�֣� O��C��O��2�֣���S��3Cl2��4H2O��SO42����8H��6Cl����2�֣����ýྻ�IJ�˿պȡ����Һ�����ڻ��������գ�����ɫ���ܲ����۲������ɫ�����������ɫ������Һ�к��м����ӣ���֮û�У�2�֣�
��2�֣� O��C��O��2�֣���S��3Cl2��4H2O��SO42����8H��6Cl����2�֣����ýྻ�IJ�˿պȡ����Һ�����ڻ��������գ�����ɫ���ܲ����۲������ɫ�����������ɫ������Һ�к��м����ӣ���֮û�У�2�֣� NH2NH3����OH����2�֣���PbS��4H2O2��PbSO4��4H2O��2�֣�
NH2NH3����OH����2�֣���PbS��4H2O2��PbSO4��4H2O��2�֣�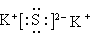 ��CO2��ֱ���ι��ۻ������ṹʽΪO��C��O��
��CO2��ֱ���ι��ۻ������ṹʽΪO��C��O�� NH2NH3����OH����
NH2NH3����OH���� ��
�� ��3�����x��3�����Է�Ӧ�Ļ�ѧ����ʽΪ2HN3��3N2+H2��
��3�����x��3�����Է�Ӧ�Ļ�ѧ����ʽΪ2HN3��3N2+H2��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
A����ⱥ��ʳ��ˮ��2Cl��+ 2H2O 2OH��+ H2��+ Cl2�� 2OH��+ H2��+ Cl2�� |
| B�������ȥˮ���е�CaCO3��CaCO3+ 2H+��Ca2+ + H2O + CO2�� |
C��Ư����Һ�ڿ�����ʧЧ��ClO��+ CO2 + H2O��HClO + HCO |
D����NaHSO4��Һ�еμ�NaHCO3��Һ��HSO + HCO + HCO �� H2O + SO �� H2O + SO + CO2�� + CO2�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������������������Fe(OH)3��3H��=Fe3����3H2O |
| B������ͭ��Һ�����ԣ�Cu2����2H2O=Cu(OH)2����2H�� |
C����̼�������Һ�мӹ���ʯ��ˮ�����ȣ� ��OH��=NH3����H2O ��OH��=NH3����H2O |
D�����ữ�ĸ��������Һ����˫��ˮ��2 ��6H����5H2O2=2Mn2����5O2����8H2O ��6H����5H2O2=2Mn2����5O2����8H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ϡ���������м��Ӧ��Fe��4H����NO3��=Fe3����NO����2H2O |
| B�������������Һ�м�����������������Һ��NH4����OH��=NH3����H2O |
| C���Ȼ�����Һ�м��������ˮ��Al3����4NH3��H2O=AlO2����4NH4����2H2O |
| D��H2C2O4ʹ����KMnO4��Һ��ɫ��5H2C2O4��6H����2MnO4��=10CO2����2Mn2����8H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
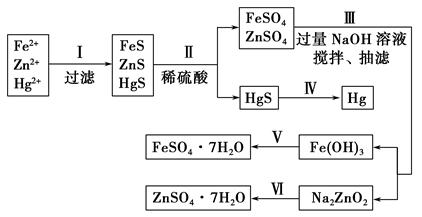
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������ˮ��Ӧ��Na��H2O��Na����OH����H2�� |
| B��������ˮ��Ӧ��Cl2��H2O��2H����Cl����ClO�� |
| C��̼��������Һ�м����������ƣ�HCO3�� ��OH���� CO2����H2O |
| D�����������к�θ�Al(OH)3��3H����Al3����3H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��H����OH��=H2O |
| B��Al3����3OH��=Al(OH)3�� |
| C��Al(OH)3��OH��=AlO2-��2H2O |
| D��NH4+��OH��=NH3��H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ͭ��ϡ���ᷴӦ��3Cu+ 8H++2NO3-=3Cu2++2NO��+4H2O |
| B��ʵ�����ô���ʯ��ϡ������ȡCO2��2H+ + CO32-��CO2��+ H2O |
| C����������ϡ���ᷴӦ��2Fe+6H+=2Fe3+ + 3H2�� |
| D������ˮ��Ӧ�� Na��H2O��Na����OH����H2�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
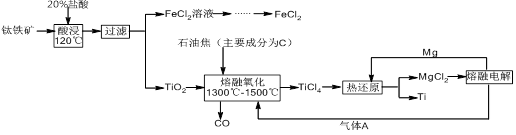
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com