
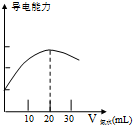
 ijѧϰС����DISϵͳ�ⶨʳ�ð״��д�������ʵ���Ũ�ȣ�����Һ�ĵ����������жϵζ��յ㣮ʵ�鲽�����£�
ijѧϰС����DISϵͳ�ⶨʳ�ð״��д�������ʵ���Ũ�ȣ�����Һ�ĵ����������жϵζ��յ㣮ʵ�鲽�����£����� ��1��������ȡҺ������ľ�ȷ���Լ�Һ�������ѡ�����������ձ���ϡ����Һ��������ƿ����һ��Ũ�ȵ���Һ��
��2���ζ�����ϴ��֮��ܱ��ϲ���ˮ����ʹ������ҺŨ���½��ݴ˷������
��3��������һˮ�ϰ���Ӧ�����κ�ˮ��
��4������ͼ����֪�����Һ����������ǿʱ������ǡ���백ˮ��Ӧ��ȫ���ֱ������Ӧ���ĵĴ�������ʵ����Ͱ�ˮ�����ʵ�������������кͷ�Ӧn��CH3COOH��=n��NH3•H2O��������⣻��ȡʳ�ð״�����û����ʳ�ð״���ϴ���״ױ�ϡ�ͣ�
��� �⣺��1���ζ��ܾ�ȷ��Ϊ0.01ml��������������ܸ�ʴ�ܣ�����Ӧѡ����ʽ�ζ�����ȡ�״ף�Ȼ��Ѱ״ף�ת�Ƶ��ձ��м�ˮϡ����Һ���ٰ���Һת��100mL����ƿ���ж��ݣ�
�ʴ�Ϊ����ʽ�ζ��ܣ��ձ�������ƿ��
��2��Ϊ��ֹ�ζ�����ϴ��֮��ܱ��ϲ���ˮ������Һϡ�ͣ�Ӧ����ʢҺ����ϴ2-3�Σ�
�ʴ�Ϊ����ˮ����ֹ��ˮ��ϡ�ͣ�
��3��������һˮ�ϰ���Ӧ���ɴ���狀�ˮ��CH3COOH+NH3•H2O=CH3COONH4+H2O��
�ʴ�Ϊ��CH3COOH+NH3•H2O=CH3COO-+NH4++H2O��
��4����״�Ũ��ΪC����Ӧ���ĵĴ�������ʵ���Ϊn��CH3COOH��=C��10.00mL��$\frac{20}{100}$��
��Ӧ���ĵİ�ˮ�����ʵ���Ϊ��n��NH3•H2O��=0.1000mol•L-1��20ml��
����ͼ����֪�����Һ����������ǿʱ������ǡ���백ˮ��Ӧ��ȫ��
����n��CH3COOH��=n��NH3•H2O����C��10.00mL��$\frac{20}{100}$=0.1000mol•L-1��20ml��C=1.000mol•L-1��
��ȡʳ�ð״�����û����ʳ�ð״���ϴ���״ױ�ϡ�ͣ��백ˮ��Ӧʱ���ĵİ�ˮ�������ƫС����������İ״�Ũ�Ȼ�ƫ�ͣ�
�ʴ�Ϊ��1.000mol•L-1��ƫ�ͣ�
���� ���⿼����Һ�����ơ��к͵ζ�ʵ�飬��Ŀ�Ѷ��еȣ�ע��ζ��ܵ�ѡ���ʹ�õ�ע����������ڿ���ѧ����ʵ�����������


 Ӣ�żƻ���ĩ����ϵ�д�
Ӣ�żƻ���ĩ����ϵ�д� ��Ӣ���㿨ϵ�д�
��Ӣ���㿨ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
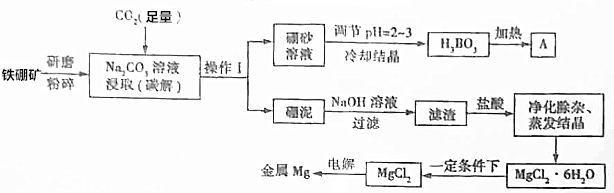
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��֪ˮ��25���95��ʱ�������ƽ��������ͼ��ʾ��
��֪ˮ��25���95��ʱ�������ƽ��������ͼ��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 1 mol�κ�����������Ϊ22.4 L | |
| B�� | 1 mol�κ������ڱ�״������ռ�������Ϊ22.4 L | |
| C�� | ��״���£�1 mol���Ȼ�̼��ռ�������22.4 L | |
| D�� | ��״���£�22.4 L���κ���������ʵ�������1 mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
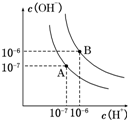
 �����£���0.10mol•L-1�������20.00mL 0.10mol•L-1��ˮ�У���Һ��pH��pOH�������������仯������ͼ��ʾ����֪��pOH=-lg c��OH-��������˵����ȷ���ǣ�������
�����£���0.10mol•L-1�������20.00mL 0.10mol•L-1��ˮ�У���Һ��pH��pOH�������������仯������ͼ��ʾ����֪��pOH=-lg c��OH-��������˵����ȷ���ǣ�������| A�� | M����ʾ��Һ��c��NH4+��+c��NH3•H2O��=c��Cl-�� | |
| B�� | N����ʾ��Һ��c��NH4+����c��Cl-�� | |
| C�� | Q�����������������ڰ�ˮ����� | |
| D�� | M���N����ʾ��Һ��ˮ�ĵ���̶���ͬ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | īˮ | B�� | Fe��OH��3���� | C�� | CuSO4��Һ | D�� | ϡ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��������� | B�� | �Ҵ������� | C�� | ��ϩ�ͱ��� | D�� | ��Ȳ�ͱ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ʪ��ұͭ��Fe+CuSO4�TFeSO4+Cu | |
| B�� | ��¯������Fe2O3+3CO�T2Fe+3CO2 | |
| C�� | ��������2KMnO4$\frac{\underline{\;\;��\;\;}}{\;}$K2MnO4+MnO2+O2�� | |
| D�� | �ƻ������飺2C2H2�� ��Ȳ��+O2$\frac{\underline{\;����\;}}{����}$2C2H4O �� �������飩 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | C3H6��C2H4 | B�� | C2H4��C2H6 | C�� | C3H8��C3H6 | D�� | C6H6��C2H2 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com