

 53���ò�ϵ�д�
53���ò�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��F>N>O | B��O>Cl>F | C��As>P>H | D��Cl>S>As |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���ڶ�����Ԫ���У��������������ڸ�ԭ�Ӻ�����Ӳ���2����ֻ��C��S |
| B�����ӻ������г��������Ӽ��⣬�����ܺ��м��Լ���Ǽ��Լ� |
| C������Ԫ���⣬��A��Ԫ�صĽ����ԱȢ�A��Ԫ�صĽ�����ǿ |
| D��H2O��D2O��Ϊͬλ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������



























| ������ | Na+ Mg2+ Al3+ Ag+ Ba2+ |
| ������ | OH- Cl- SO42- SO32- CO32- |

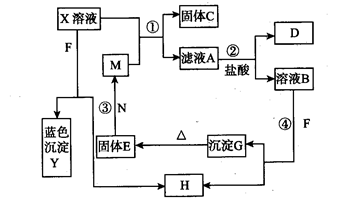
 Ϊ�˼������е����ӣ��ֽ�������ʵ�飬�Ը���ʵ�������Ҫ����ա�
Ϊ�˼������е����ӣ��ֽ�������ʵ�飬�Ը���ʵ�������Ҫ����ա�
 (1)ȡ�����ð�ɫ�����ˮ�ܽ⣬���õ���ɫ��Һ����pH��ֽ��⣬��Һ��pHΪ13����X��һ�������ڵ�������_____________��
(1)ȡ�����ð�ɫ�����ˮ�ܽ⣬���õ���ɫ��Һ����pH��ֽ��⣬��Һ��pHΪ13����X��һ�������ڵ�������_____________��
 (2)����Һ�еμ�������Һ���տ�ʼ���������г������ɣ������μӳ�����ʧ��������ɫ��ζ�����ݳ�������(1)��(2)�ɵó��Ľ����ǣ�X��һ�����ڵ�������____________���ֿ�ȷ��X��һ�������ڵ�������________________��
(2)����Һ�еμ�������Һ���տ�ʼ���������г������ɣ������μӳ�����ʧ��������ɫ��ζ�����ݳ�������(1)��(2)�ɵó��Ľ����ǣ�X��һ�����ڵ�������____________���ֿ�ȷ��X��һ�������ڵ�������________________��
 (3)�����X�����������֣��������(�û�ѧʽ��ʾ��д��һ�鼴��) ________________��
(3)�����X�����������֣��������(�û�ѧʽ��ʾ��д��һ�鼴��) ________________��
 (4)�д���������Ӽ����鷽���ǣ�
(4)�д���������Ӽ����鷽���ǣ�| �д���������� | ���鷽�� |
            | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
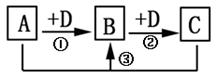
 A)ʱ��AΪ�� ��
A)ʱ��AΪ�� ���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com