



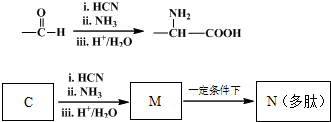
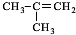 ���巢���ӳɷ�Ӧ����A��AΪ
���巢���ӳɷ�Ӧ����A��AΪ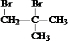 ��A����������ˮ��Һ�����������·���ˮ�ⷴӦ����B��BΪHOCH2C��OH����CH3��2��B����������C��C��������D�������֪��֪CΪOHCCH2C��OH����CH3��2����DΪHOOCC��OH����CH3��2���ۺϷ���E��F��Ӧ��F�Ľṹ��ʽ�к���̼̼˫����֪D��Ũ��������������E��E�뻷�����鷴Ӧ����F�����F�Ľṹ��֪��EΪCH2=C��CH3��COOH��F��ֻ��ͨ���Ӿ۷�Ӧ���ɸ߷��ӻ�����G���ݴ˽��1������6����CΪ
��A����������ˮ��Һ�����������·���ˮ�ⷴӦ����B��BΪHOCH2C��OH����CH3��2��B����������C��C��������D�������֪��֪CΪOHCCH2C��OH����CH3��2����DΪHOOCC��OH����CH3��2���ۺϷ���E��F��Ӧ��F�Ľṹ��ʽ�к���̼̼˫����֪D��Ũ��������������E��E�뻷�����鷴Ӧ����F�����F�Ľṹ��֪��EΪCH2=C��CH3��COOH��F��ֻ��ͨ���Ӿ۷�Ӧ���ɸ߷��ӻ�����G���ݴ˽��1������6����CΪ �������֪��Ӧ�ƶ�MӦΪ��CH3��2C��OH��CH��NH2��COOH������֪����N���ݴ˽��
�������֪��Ӧ�ƶ�MӦΪ��CH3��2C��OH��CH��NH2��COOH������֪����N���ݴ˽��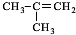 ���巢���ӳɷ�Ӧ����A��AΪ
���巢���ӳɷ�Ӧ����A��AΪ ��A����������ˮ��Һ�����������·���ˮ�ⷴӦ����B��BΪHOCH2C��OH����CH3��2��B����������C��C��������D�������֪��֪CΪOHCCH2C��OH����CH3��2����DΪHOOCC��OH����CH3��2���ۺϷ���E��F��Ӧ��F�Ľṹ��ʽ�к���̼̼˫����֪D��Ũ��������������E��E�뻷�����鷴Ӧ����F�����F�Ľṹ��֪��EΪCH2=C��CH3��COOH��F��ֻ��ͨ���Ӿ۷�Ӧ���ɸ߷��ӻ�����G��
��A����������ˮ��Һ�����������·���ˮ�ⷴӦ����B��BΪHOCH2C��OH����CH3��2��B����������C��C��������D�������֪��֪CΪOHCCH2C��OH����CH3��2����DΪHOOCC��OH����CH3��2���ۺϷ���E��F��Ӧ��F�Ľṹ��ʽ�к���̼̼˫����֪D��Ũ��������������E��E�뻷�����鷴Ӧ����F�����F�Ľṹ��֪��EΪCH2=C��CH3��COOH��F��ֻ��ͨ���Ӿ۷�Ӧ���ɸ߷��ӻ�����G�� ��A����������ˮ��Һ�����������·���ˮ�ⷴӦ����B���ʴ�Ϊ��ˮ�ⷴӦ����ȡ����Ӧ����
��A����������ˮ��Һ�����������·���ˮ�ⷴӦ����B���ʴ�Ϊ��ˮ�ⷴӦ����ȡ����Ӧ���� ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��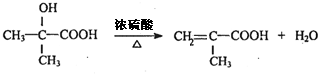 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��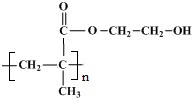 ��
�� ��
��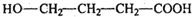 ��
��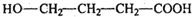 ��
�� �������֪��Ӧ�ƶ�MӦΪ��CH3��2C��OH��CH��NH2��COOH������֪���ɶ���N�ķ�Ӧ����ʽΪ��
�������֪��Ӧ�ƶ�MӦΪ��CH3��2C��OH��CH��NH2��COOH������֪���ɶ���N�ķ�Ӧ����ʽΪ�� ��
�� ��
�� ��ȩ���ɷ����ӳɷ�Ӧ���ǻ�����λH�ɷ�����ȥ��Ӧ����b��ȷ��
��ȩ���ɷ����ӳɷ�Ӧ���ǻ�����λH�ɷ�����ȥ��Ӧ����b��ȷ��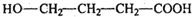 �����ǻ����ڵڶ���C�ϡ�������C�ϣ�����3�����ϣ���e����
�����ǻ����ڵڶ���C�ϡ�������C�ϣ�����3�����ϣ���e����


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����Һ��������Na2CO3��NaOH |
| B����Һ�е�����Ũ�ȣ�c��Na+����c��HCO3-����c��CO32-����c��OH-����c��H+�� |
| C����Һ�е�����Ũ�ȣ�c��Na+����c��HCO3-��=c��CO32-����c��OH-����c��H+�� |
| D����Һ�е�����Ũ�ȣ�c��Na+����c��CO32-����c��HCO3-����c��OH-����c��H+�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 W��X��Y��Z�����ֳ����Ķ�����Ԫ�أ���ԭ�Ӱ뾶��ԭ�������仯��ͼ��ʾ����֪W��һ�ֺ��ص�������Ϊ18��������Ϊ10��X��Neԭ�ӵĺ�����������1��Y�ĵ�����һ�ֳ����İ뵼����ϣ�Z��ԭ�Ӱ뾶��ͬ��������Ԫ������С��
W��X��Y��Z�����ֳ����Ķ�����Ԫ�أ���ԭ�Ӱ뾶��ԭ�������仯��ͼ��ʾ����֪W��һ�ֺ��ص�������Ϊ18��������Ϊ10��X��Neԭ�ӵĺ�����������1��Y�ĵ�����һ�ֳ����İ뵼����ϣ�Z��ԭ�Ӱ뾶��ͬ��������Ԫ������С���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| �� ���� |
IA | IIA | IIIA | IVA | VA | VIA | VIIA | 0 |
| 2 | H | A | ||||||
| 3 | B | C | D | E | ||||
| 4 | F | G |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��HMnO4 |
| B��H2SeO3 |
| C��H3BO3 |
| D��H3PO4 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com