
���磺RCH=CHR��![]() RCHO+R��CHO
RCHO+R��CHO
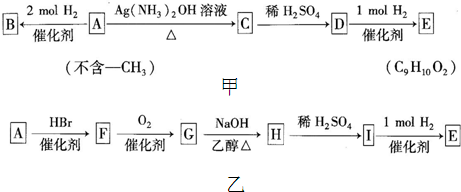
���п�ͼ�з����ڵ���ĸ������һ�ַ����廯�������B�Ľṹ�в�������D�ķ���ʽΪC9H8O2��Aת��ΪBʱ��A��H�������ʵ���֮��Ϊ1��2(�������ʼ�ת�����е���Ҫ����)��

��ش��������⣺
(1)A�ķ���ʽΪ______________��F�Ľṹ��ʽΪ______________��
A![]() C�Ļ�ѧ����ʽΪ______________________________________��
C�Ļ�ѧ����ʽΪ______________________________________��
(2)B��ijЩͬ���칹���ܸ�Ũ��ˮ��Ӧ������1 molijͬ���칹���Ũ��ˮ��Ӧʱ��������3 mol Br2���Ҹ�ͬ���칹������к���һ����������ṹ��ʽΪ
_______________________________________________________________��
(3)A��һ�ֿ�����Ϊҩ����л����A��һ����������������Եõ������л���X��Y������Y�Ƕ�Ԫ�ᡣ��X�Ľṹ��ʽΪ______________��Y�Ľṹ��ʽΪ______________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| һ������ |





 ����дһ�֣�
����дһ�֣� ����дһ�֣�
����дһ�֣�



�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| һ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��16�֣���֪ϩ����һ�������¿ɱ�ǿ������������ȩ�����磺
RCH=CHR��![]() RCHO + R��CHO
RCHO + R��CHO
��A��������ͼ��ʾ��ϵ��

��1��A�Ļ�ѧʽΪ________________��E�Ľṹ��ʽΪ_____________________��
A��C�Ļ�ѧ��Ӧ����ʽΪ��_____________________________________��
��2����B��һ��ͬ���칹�����ˮ��Ӧ���ɰ�ɫ��������1mol��ͬ���칹�����ˮ��Ӧʱ��������3mol Br2������ϸ�������ͬ���칹����_______�֣�д����������һ�ֵĽṹ��ʽ��_______________________��
��3��A��һ�ֿ�����Ϊҩ����л����A��һ���������������Եõ������л���X��Y��X���ڷ����廯�������ʳƷ���漰Ⱦ�ϡ�ҩ�������о�����Ҫ���ã���X�Ľṹ��ʽΪ��___________________________��
��4����A��E������ͨ������;����
![]()
����Ʋ���A��F��Ŀ����_______________��
��д��G��H�Ļ�ѧ��Ӧ����ʽ��_______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��߿���ѧ������ϰ��C9���л���ѧ�������������棩 ���ͣ������
 RCHO+R��CHO��
RCHO+R��CHO��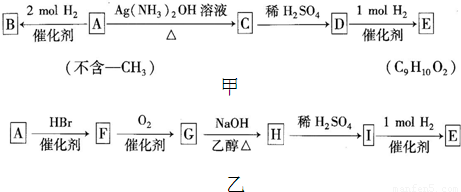
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com