
ʵ������2.0 mol/L NaCl��Һ����100mL 0.50 mol/L NaCl��Һ��
��1������������Һ����Ҫ2.0 mol/L NaCl��Һ mL��
��2������������Һ����Ҫ����������ͷ�ι��⣬����Ҫ�IJ����������ձ�����Ͳ�� __________________��20g NaOH�����ܽ� �����Ƴ�100mL��Һ�����������ʵ���Ϊ ��ȡ��10mL����Һ���������ʵ���Ũ��Ϊ ��
��3�������ƹ����У�������������ȷ�����в����У�������Ũ��ƫ�ߵ��� ������ţ�: ��
�� ����ʱ������ˮ�����̶��ߣ����ý�ͷ�ι�����
��ת����Һǰ������ƿ�к������� ˮ
�۶���ҡ�Ⱥ���Һ����ڿ̶��ߣ����ý�ͷ�ιܼ�����ˮ���̶���
�ܶ���ʱ�����ӿ̶���
��5�֣���1��25 mL ��2��100ml����ƿ��0.5mol��5 mol/L ��3����
���������������1�����Ȼ��Ƶ�ϡ�����У������Dz���ģ�������Ҫ�Ȼ�����Һ�������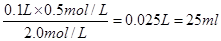 ��
��
��2������������������֪����ȱ��100ml����ƿ��20g NaOH��������ʵ�����20g��40g/mol��0.5mol������Ũ����0.5mol��0.1L��5mol/L��
��3������n��c��V��֪���������ʱ������ˮ�����̶��ߣ����ý�ͷ�ι������������ʼ��٣�Ũ��ƫ�ͣ�ת����Һǰ������ƿ�к�������ˮ�����ʺ���Һ��������䣬Ũ�Ȳ��䣻����ҡ�Ⱥ���Һ����ڿ̶��ߣ����ý�ͷ�ιܼ�����ˮ���̶��ߣ�����Һ������ӣ�Ũ��ƫ�ͣ�����ʱ�����ӿ̶��ߣ�����Һ������٣�Ũ��ƫ�ߣ���ѡ�ܡ�
���㣺����һ�����ʵ���Ũ����Һ�����ƺͼ���
�������������е��Ѷȵ����⣬�����ڿ������֪ʶ��ͬʱ�����������������Ľ��ⷽ��ָ��������������ѧ������˼ά������������ѵ�����������������ѧ����Ҫ��ȷ�������ʵ���Ũ����Һʱ��Ҫע�����cB��nB/V�ɵã�һ�����ʵ���Ũ����Һ���Ƶ����������ʵ����ʵ�����B����Һ�����V����ġ�������ʱ���ؼ�Ҫ�����ƹ�������������V�����ı仯��������һ�����ʵ���Ũ����Һʱ����nB������ֵС����V������ֵ��ʱ������ʹ������ҺŨ��ƫС����nB������ֵ��V������ֵСʱ������ʹ������ҺŨ��ƫ��


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ���㶫ʡ2009�������ѧ������У�¿������࣭�绯ѧ(2) ���ͣ�021
|
��֪Ǧ���طŵ�ʱ�������·�Ӧ�� ������Pb��SO42����PbSO4��2e�� ������PbO2��4H+��SO42����2e����PbSO4��2H2O ʵ������Ǧ��������Դ���ö��Ե缫���CuSO4��Һ������������2.4 gͭʱ��Ǧ����������H2SO4���ʵ��������� | |
A�� |
0.075 mol |
B�� |
0.050 mo |
C�� |
0.20 mol |
D�� |
0.40 mol |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com