


 ЗДгІЕФЛЏбЇЗНГЬЪНЃК
ЗДгІЕФЛЏбЇЗНГЬЪНЃК

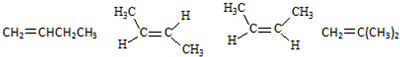
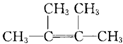 ЃЌAКЭТШЦјЗЂЩњМгГЩЗДгІЩњГЩBЃЌBЕФНсЙЙМђЪНЮЊЃКЃЈCH3ЃЉ2 CClCClЃЈCH3ЃЉ2ЃЌBКЭЧтбѕЛЏФЦЕФДМШмвКЗЂЩњЯћШЅЗДгІЩњГЩCЃЌCЕФНсЙЙМђЪНЮЊЃКCH2=CЃЈCH3ЃЉCЃЈCH3ЃЉ=CH2ЃЌCКЭфхЗЂЩњ1ЃЌ2-МгГЩЗДгІЩњГЩD1ЃЌD1ЕФНсЙЙМђЪНЮЊЃКCH2BrCЃЈCH3ЃЉBrCЃЈCH3ЃЉ=CH2ЃЌD1КЭЧтбѕЛЏФЦЕФЫЎШмвКЗЂЩњШЁДњЗДгІЩњГЩE1ЃЌE1ЕФНсЙЙМђЪНЮЊЃКCH2OHCЃЈCH3ЃЉOHCЃЈCH3ЃЉ=CH2ЃЌD1ЁЂD2ЛЅЮЊЭЌЗжвьЙЙЬхЃЌЙЪЗДгІЂмЗЂЩњ1ЃЌ4-МгГЩЃЌD2ЮЊCH2BrCЃЈCH3ЃЉ=CЃЈCH3ЃЉCH2BrЃЌD2дкЧтбѕЛЏФЦЫЎШмвКжаЗЂЩњЫЎНтЗДгІЩњГЩE2ЃЌE2ЮЊHOCH2CЃЈCH3ЃЉ=CЃЈCH3ЃЉCH2OHЃЌИљОнгаЛњЮяЕФНсЙЙКЭаджЪНтД№ЃЎ
ЃЌAКЭТШЦјЗЂЩњМгГЩЗДгІЩњГЩBЃЌBЕФНсЙЙМђЪНЮЊЃКЃЈCH3ЃЉ2 CClCClЃЈCH3ЃЉ2ЃЌBКЭЧтбѕЛЏФЦЕФДМШмвКЗЂЩњЯћШЅЗДгІЩњГЩCЃЌCЕФНсЙЙМђЪНЮЊЃКCH2=CЃЈCH3ЃЉCЃЈCH3ЃЉ=CH2ЃЌCКЭфхЗЂЩњ1ЃЌ2-МгГЩЗДгІЩњГЩD1ЃЌD1ЕФНсЙЙМђЪНЮЊЃКCH2BrCЃЈCH3ЃЉBrCЃЈCH3ЃЉ=CH2ЃЌD1КЭЧтбѕЛЏФЦЕФЫЎШмвКЗЂЩњШЁДњЗДгІЩњГЩE1ЃЌE1ЕФНсЙЙМђЪНЮЊЃКCH2OHCЃЈCH3ЃЉOHCЃЈCH3ЃЉ=CH2ЃЌD1ЁЂD2ЛЅЮЊЭЌЗжвьЙЙЬхЃЌЙЪЗДгІЂмЗЂЩњ1ЃЌ4-МгГЩЃЌD2ЮЊCH2BrCЃЈCH3ЃЉ=CЃЈCH3ЃЉCH2BrЃЌD2дкЧтбѕЛЏФЦЫЎШмвКжаЗЂЩњЫЎНтЗДгІЩњГЩE2ЃЌE2ЮЊHOCH2CЃЈCH3ЃЉ=CЃЈCH3ЃЉCH2OHЃЌИљОнгаЛњЮяЕФНсЙЙКЭаджЪНтД№ЃЎ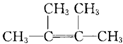 ЃЌAКЭТШЦјЗЂЩњМгГЩЗДгІЩњГЩBЃЌBЕФНсЙЙМђЪНЮЊЃКЃЈCH3ЃЉ2 CClCClЃЈCH3ЃЉ2ЃЌBКЭЧтбѕЛЏФЦЕФДМШмвКЗЂЩњЯћШЅЗДгІЩњГЩCЃЌCЕФНсЙЙМђЪНЮЊЃКCH2=CЃЈCH3ЃЉCЃЈCH3ЃЉ=CH2ЃЌCКЭфхЗЂЩњ1ЃЌ2-МгГЩЗДгІЩњГЩD1ЃЌD1ЕФНсЙЙМђЪНЮЊЃКCH2BrCЃЈCH3ЃЉBrCЃЈCH3ЃЉ=CH2ЃЌD1КЭЧтбѕЛЏФЦЕФЫЎШмвКЗЂЩњШЁДњЗДгІЩњГЩE1ЃЌE1ЕФНсЙЙМђЪНЮЊЃКCH2OHCЃЈCH3ЃЉOHCЃЈCH3ЃЉ=CH2ЃЌD1ЁЂD2ЛЅЮЊЭЌЗжвьЙЙЬхЃЌЙЪЗДгІЂмЗЂЩњ1ЃЌ4-МгГЩЃЌD2ЮЊCH2BrCЃЈCH3ЃЉ=CЃЈCH3ЃЉCH2BrЃЌD2дкЧтбѕЛЏФЦЫЎШмвКжаЗЂЩњЫЎНтЗДгІЩњГЩE2ЃЌE2ЮЊHOCH2CЃЈCH3ЃЉ=CЃЈCH3ЃЉCH2OHЃЌ
ЃЌAКЭТШЦјЗЂЩњМгГЩЗДгІЩњГЩBЃЌBЕФНсЙЙМђЪНЮЊЃКЃЈCH3ЃЉ2 CClCClЃЈCH3ЃЉ2ЃЌBКЭЧтбѕЛЏФЦЕФДМШмвКЗЂЩњЯћШЅЗДгІЩњГЩCЃЌCЕФНсЙЙМђЪНЮЊЃКCH2=CЃЈCH3ЃЉCЃЈCH3ЃЉ=CH2ЃЌCКЭфхЗЂЩњ1ЃЌ2-МгГЩЗДгІЩњГЩD1ЃЌD1ЕФНсЙЙМђЪНЮЊЃКCH2BrCЃЈCH3ЃЉBrCЃЈCH3ЃЉ=CH2ЃЌD1КЭЧтбѕЛЏФЦЕФЫЎШмвКЗЂЩњШЁДњЗДгІЩњГЩE1ЃЌE1ЕФНсЙЙМђЪНЮЊЃКCH2OHCЃЈCH3ЃЉOHCЃЈCH3ЃЉ=CH2ЃЌD1ЁЂD2ЛЅЮЊЭЌЗжвьЙЙЬхЃЌЙЪЗДгІЂмЗЂЩњ1ЃЌ4-МгГЩЃЌD2ЮЊCH2BrCЃЈCH3ЃЉ=CЃЈCH3ЃЉCH2BrЃЌD2дкЧтбѕЛЏФЦЫЎШмвКжаЗЂЩњЫЎНтЗДгІЩњГЩE2ЃЌE2ЮЊHOCH2CЃЈCH3ЃЉ=CЃЈCH3ЃЉCH2OHЃЌ
| ||
| Ёї |
| ||
| Ёї |
 ЃЌ
ЃЌ ЃЛ
ЃЛ ЃЌ
ЃЌ ЃЎ
ЃЎ

 ПЮЬУШЋНтзжДЪОфЖЮЦЊеТЯЕСаД№АИ
ПЮЬУШЋНтзжДЪОфЖЮЦЊеТЯЕСаД№АИ ВНВНИпПкЫуЬтПЈЯЕСаД№АИ
ВНВНИпПкЫуЬтПЈЯЕСаД№АИ
| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
 ЃЈ2010?ФЯПЊЧјвЛФЃЃЉШчЭМЫљЪОЃЌНЋДПFeАєКЭЪЏФЋАєВхШы1L БЅКЭNaClШмвКжаЃЎЯТСаЫЕЗЈе§ШЗЕФЪЧЃЈЁЁЁЁЃЉ
ЃЈ2010?ФЯПЊЧјвЛФЃЃЉШчЭМЫљЪОЃЌНЋДПFeАєКЭЪЏФЋАєВхШы1L БЅКЭNaClШмвКжаЃЎЯТСаЫЕЗЈе§ШЗЕФЪЧЃЈЁЁЁЁЃЉВщПДД№АИКЭНтЮі>>
АйЖШжТаХ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com