
 �ش�
�ش� ����Ľṹ��ʽ��_____________________��
����Ľṹ��ʽ��_____________________�� ���ɶ���ܲ��¿�ֱͨ��Уϵ�д�
���ɶ���ܲ��¿�ֱͨ��Уϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
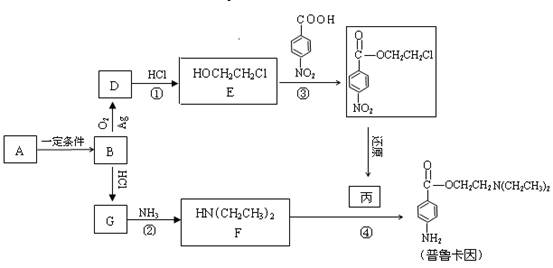
 ����Ӧ�IJ��ֲ�����ʡ�ԣ�����֪AΪ����Ԫ�أ�B�ڳ�����ΪҺ�壬E��GΪ��ȼ�����壬RΪ����ɫ
����Ӧ�IJ��ֲ�����ʡ�ԣ�����֪AΪ����Ԫ�أ�B�ڳ�����ΪҺ�壬E��GΪ��ȼ�����壬RΪ����ɫ ���壬K�����ʵ����ɫ��L��P����������Һ����������Ӧ������S�ķ���ʽΪC4H4O4�����������һ����Ԫ����TΪ�߷��ӻ����
���壬K�����ʵ����ɫ��L��P����������Һ����������Ӧ������S�ķ���ʽΪC4H4O4�����������һ����Ԫ����TΪ�߷��ӻ����
 ��Ư���� B�������� C����ԭ�� D������
��Ư���� B�������� C����ԭ�� D�������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
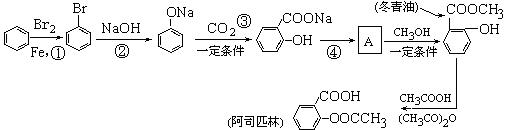
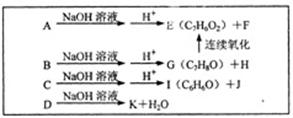
 ��1��д������ת���ڵĻ�ѧ��Ӧ����ʽ��
��1��д������ת���ڵĻ�ѧ��Ӧ����ʽ�� ___________________________________________��
___________________________________________���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
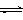 CH3OH(g)��H2O(g) ����H����49.0kJ/mol�����CO2��CH3OH(g)��Ũ����ʱ��仯��ͼ��ʾ��
CH3OH(g)��H2O(g) ����H����49.0kJ/mol�����CO2��CH3OH(g)��Ũ����ʱ��仯��ͼ��ʾ��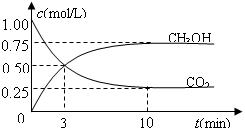
| A�������¶� | B������He(g)��ʹ��ϵѹǿ���� |
| C����H2O(g)����ϵ�з��� | D���ٳ���1mol H2 |
 ����CO����Ⱦ��������
����CO����Ⱦ�������� ���Ƿ���в�˵�����ɣ�����������������������������������������������������������������
���Ƿ���в�˵�����ɣ������������������������������������������������������������������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
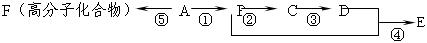
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
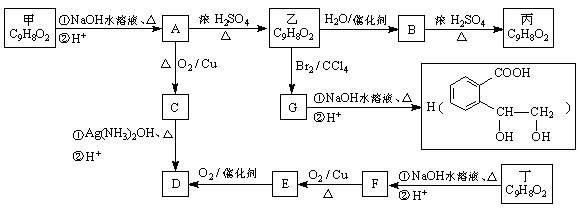
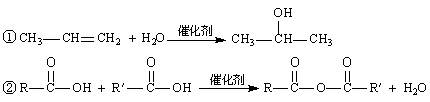

 �ṹ)����X�Ľṹ��ʽΪ (��дһ��)��
�ṹ)����X�Ľṹ��ʽΪ (��дһ��)��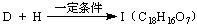 �������������I���� ��
�������������I���� ���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com