

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ���������������
���������������| ��ѧ�� | Si-O | Si-Cl | H-H | H-Cl | Si-Si | Si-C | O=O |
| ����/kJ?mol-1 | 460 | 360 | 436 | 431 | 176 | 347 | 498 |
| ||
| ʵ����� | ��ʼ�¶�t1/�� | ��ֹ�¶�t2/�� | �²t2-t1�� /�� | ||
| ���� | NaOH��Һ | ƽ��ֵ | |||
| 1 | 25.1 | 24.9 | 25.0 | 31.6 | 6.6 |
| 2 | 25.1 | 25.1 | 31.8 | 31.8 | 6.7 |
| 3 | 25.1 | 25.1 | 25.1 | 31.9 | 6.8 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�013
���и���ѧ��Ӧ�з�Ӧ����������
[����]
A��1.01��![]() Pa��25��ʱ1gþͶ��100mLŨ��Ϊ2mol/L��
Pa��25��ʱ1gþͶ��100mLŨ��Ϊ2mol/L��![]() ��Һ��
��Һ��
B��1.01��![]() Pa��25��ʱ1gþͶ��100mLŨ��Ϊ2mol/L��������Һ��
Pa��25��ʱ1gþͶ��100mLŨ��Ϊ2mol/L��������Һ��
C��1.01��![]() Pa��50��ʱ1gþͶ��100mLŨ��Ϊ3mol/L��
Pa��50��ʱ1gþͶ��100mLŨ��Ϊ3mol/L��![]() ��Һ��
��Һ��
D��1.01��![]() Pa��50��ʱ1gþͶ��100mLŨ��Ϊ4mol/L��������Һ��
Pa��50��ʱ1gþͶ��100mLŨ��Ϊ4mol/L��������Һ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�����л�ѧ�������ᣩ��������ʦ�������Ծ��� ���ͣ�013
���и���ѧ��Ӧ�з�Ӧ����������
[����]
A��1.01��![]() Pa��25��ʱ1gþͶ��100mLŨ��Ϊ2mol/L��
Pa��25��ʱ1gþͶ��100mLŨ��Ϊ2mol/L��![]() ��Һ��
��Һ��
B��1.01��![]() Pa��25��ʱ1gþͶ��100mLŨ��Ϊ2mol/L��������Һ��
Pa��25��ʱ1gþͶ��100mLŨ��Ϊ2mol/L��������Һ��
C��1.01��![]() Pa��50��ʱ1gþͶ��100mLŨ��Ϊ3mol/L��
Pa��50��ʱ1gþͶ��100mLŨ��Ϊ3mol/L��![]() ��Һ��
��Һ��
D��1.01��![]() Pa��50��ʱ1gþͶ��100mLŨ��Ϊ4mol/L��������Һ��
Pa��50��ʱ1gþͶ��100mLŨ��Ϊ4mol/L��������Һ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪����1���������������������¿�ˮ������A�� ����B��C9H18SNCl����C��H3PO4��
����B��C9H18SNCl����C��H3PO4��
��2������ũҩ����A��Ϊͬ���칹�壬�������������¹�ϵ��
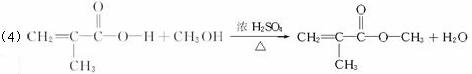
��ش��������⣺
��1��A�ķ���ʽΪ___________________________________________��
��2���Ľṹ��ʽΪ_________________________________________________��
��3��д�����и����ķ�Ӧ���ͣ���___________����___________��
��4��д�������ɶ��Ļ�ѧ��Ӧ����ʽ____________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com