

��1������I=0.21 A�ĵ������60���Ӻ��ͭƬA������������0.25 g����ͼ4��4װ���е�X��Ӧ��ֱ�����__________�����������ǵ��ص�__________����
��2������ͭƬB������__________��ѡ����ӡ������١����䡱��
��3����ʽ����ʵ���õİ����ӵ�����NA������֪���ӵ����e��=1.60��10��19 C��
��������1���ɵ��ͭ�����֪ʶ����֪ͭƬA���������ӣ�˵��A����������֮������X��Ӧ��ֱ����ĸ���������
��2����B��������������Ӧ��Cu��2e��====Cu2+���ʵ���ͭƬB���������٣����ٵ��������ܡ���A�ϡ�
��3��ÿ����1 mol Cu����Ҫת��2 mol e����������0.25 g Cu����ת��2��![]() =0.0078 mol e����
=0.0078 mol e����
������ѧ֪ʶ֪����ϵ��ͨ���ĵ��ӵ����ʵ���Ϊn��e����=![]()
![]() =0.0078 mol��
=0.0078 mol��
NA=0.21 A��60��![]() =6.0��1023 mol��1
=6.0��1023 mol��1
�𰸣���1���� ��
��2������
��3��NA=![]() =6.0��1023 mol��1
=6.0��1023 mol��1


 ���Ž�����ٰθ��νӹ㶫���������ϵ�д�
���Ž�����ٰθ��νӹ㶫���������ϵ�д� �����������ҵ�������������ϵ�д�
�����������ҵ�������������ϵ�д� ѧ���������ν��Ͼ���ѧ������ϵ�д�
ѧ���������ν��Ͼ���ѧ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

(1)����I=
(2)����ͭƬB������____________��(����ӡ������١����䡱)
(3)��ʽ����ʵ���õİ����ӵ�����NA(��֪������e=1.60��10
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ�ǿ����ڲ��������ӵ�������װ��ʾ��ͼ������A��B�����鴿ͭƬ������CuSO4ϡ��Һ�У�ͭƬ���������������������˷ֱ�ΪX��Y��
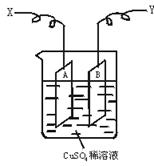
(1)����I=0.21 A�ĵ������60 min���ͭƬA������������0.25 g����ͼװ���е�X��Ӧ��ֱ�����__________�����������ǵ��ص�__________����
(2)����ͭƬB������__________��(�����ӡ������١����䡱)
(3)��ʽ����ʵ���õİ����ӵ�����NA��(��֪���ӵ���e=1.60��10��19C)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ�ǿ����ڲ��������ӵ�������װ��ʾ��ͼ������A��B�����鴿ͭƬ������CuSO4ϡ��Һ�У�ͭƬ���������������������˷ֱ�ΪX��Y��
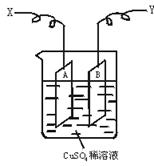
(1)����I=0.21 A�ĵ������60 min���ͭƬA������������0.25 g����ͼװ���е�X��Ӧ��ֱ�����__________�����������ǵ��ص�__________����
(2)����ͭƬB������__________��(�����ӡ������١����䡱)
(3)��ʽ����ʵ���õİ����ӵ�����NA��(��֪���ӵ���e=1.60��10��19C)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��0108 �¿��� ���ͣ������
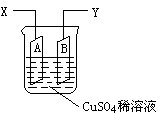
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com