
ŌŚČŻ»żĻąĶ¬µÄĆܱÕČŻĘ÷ÖŠ£¬·Ö±š³äČėµČĮæµÄµŖĘųŗĶĒāĘų£¬ŌŚ²»Ķ¬ĪĀ¶ČĻĀ·¢Éś·“Ó¦£ŗN2(g)£«3H2(g)
 2NH3(g)£¬²¢·Ö±šŌŚ²»Ķ¬µÄŹ±¼äÄŚ²ā¶ØĘäÖŠNH3µÄÖŹĮæ·ÖŹż(yÖįĖł±ķŹ¾µÄ)£¬»ę³ÉĶ¼ĻóČēĶ¼ĖłŹ¾£¬Ēė»Ų“š£ŗ
2NH3(g)£¬²¢·Ö±šŌŚ²»Ķ¬µÄŹ±¼äÄŚ²ā¶ØĘäÖŠNH3µÄÖŹĮæ·ÖŹż(yÖįĖł±ķŹ¾µÄ)£¬»ę³ÉĶ¼ĻóČēĶ¼ĖłŹ¾£¬Ēė»Ų“š£ŗ
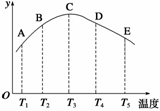
(1)A”¢B”¢C”¢D”¢EĪåµćÖŠ£¬æĻ¶ØĪ““ļĘ½ŗāµÄµćŹĒ
________________________________________________________________________ӣ
(2)“ĖæÉÄę·“Ó¦µÄÕż·“Ó¦ŹĒ__________ČČ·“Ó¦”£
(3)ACĒśĻߏĒŌöŗÆŹżĒśĻߣ¬CEĒśĻߏĒ¼õŗÆŹżĒśĻߣ¬ŹŌ“Ó»Æѧ·“Ó¦ĖŁĀŹŗĶ»ÆŃ§Ę½ŗāµÄ½Ē¶Č·ÖĪö£¬²¢ĖµĆ÷ĄķÓÉ
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________ӣ
(1)Aµć”¢Bµć””(2)·Å””(3)ACĒśĻߏĒŌöŗÆŹżĒśĻߣ¬ĪĀ¶ČÉżøߣ¬·“Ó¦ĖŁĀŹ¼Óæģ£¬Éś³ÉNH3µÄÖŹĮæ·ÖŹżŌö¶ą£»CEĒśĻߏĒ¼õŗÆŹżĒśĻߣ¬“Ė·“Ó¦µÄÕż·“Ó¦ŹĒ·ÅČČ·“Ó¦£¬“ļµ½Ę½ŗāŗó£¬ĪĀ¶ČÉżøߣ¬Ę½ŗāĻņÄę·“Ó¦·½ĻņŅĘ¶Æ£¬NH3µÄÖŹĮæ·ÖŹż½µµĶ
½āĪö””(1)“ÓĶ¼Ļó֊擳öCµćŹ±£¬NH3µÄÖŹĮæ·ÖŹż×īøߣ¬ĖµĆ÷A”¢BĮ½µć¾łĪ““ļµ½Ę½ŗā£»
(2)“ļµ½Ę½ŗā(Cµć)ŗó£¬ÉżøßĪĀ¶Č£¬NH3µÄÖŹĮæ·ÖŹż½µµĶ£¬ĖµĆ÷“Ė·“Ó¦µÄÕż·“Ó¦·ÅČČ”£


 æŚĖćĢāæؼÓÓ¦ÓĆĢā¼ÆѵĻµĮŠ“š°ø
æŚĖćĢāæؼÓÓ¦ÓĆĢā¼ÆѵĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
³£ĪĀĻĀ£¬ĻĀĮŠø÷×éĪļÖŹÖŠ£¬Y¼ČÄÜÓėX·“Ó¦ÓÖÄÜÓėZ·“Ó¦µÄŹĒ
| X | Y | Z | |
| ¢Ł | NaOHČÜŅŗ | Al(OH)3 | Ļ”ĮņĖį |
| ¢Ś | KOHČÜŅŗ | SiO2 | Ļ”ŃĪĖį |
| ¢Ū | O2 | N2 | H2 |
| ¢Ü | FeCl3ČÜŅŗ | Cu | ÅØĻõĖį |
A£®¢Ł¢Ū B£®¢Ł¢Ü C£®¢Ś¢Ü D£®¢Ś¢Ū
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠŠšŹöÖŠ£¬²»ÕżČ·µÄŹĒ(””””)
A£®ŌŚĖ®ČÜŅŗĄļŗĶČŪȌדĢ¬ĻĀ¾ł²»µ¼µēµÄ»ÆŗĻĪļ£¬½Š×ö·Ēµē½āÖŹ
B£®µē½āÖŹ”¢·Ēµē½āÖŹ¶¼Öø»ÆŗĻĪļ¶ųŃŌ£¬µ„ÖŹ²»ŌŚ“Ė·¶³ė
C£®ŌŚĖ®ÖŠµ¼µēµÄĪļÖŹŅ»¶ØŹĒµē½āÖŹ
D£®Ė®ŹĒ¼«ČõµÄµē½āÖŹ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
Äܹ»Ź¹ŗĻ³É°±·“Ó¦½ųŠŠµÄ³Ģ¶ČŌö“óµÄ“ėŹ©ŹĒ(””””)””””””””””””””””””””””””””””””””””””
A£®ÉżøßĪĀ¶Č B£®½µµĶŃ¹Ēæ C£®Ź¹ÓĆ“ß»Æ¼Į D£®²»¶ĻŅĘČ„NH3
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŌŚN2(g)£«3H2(g)
 2NH3(g)””¦¤H<0µÄĘ½ŗāĢåĻµÖŠ£¬µ±·ÖĄė³öNH3Ź±£¬ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ(””””)
2NH3(g)””¦¤H<0µÄĘ½ŗāĢåĻµÖŠ£¬µ±·ÖĄė³öNH3Ź±£¬ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ(””””)
A.øıäĢõ¼žŗóĖŁĀŹ”ŖŹ±¼äĶ¼ĻóČēÓŅĶ¼£ŗ
B£®“Ė¹ż³ĢÖŠQ>K
C£®Ę½ŗāĢåĻµÖŠNH3µÄŗ¬ĮæŌö“ó
D£®N2µÄ×Ŗ»ÆĀŹŌö“ó
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻÖĻņŗ¬AgBrµÄ±„ŗĶČÜŅŗÖŠ£ŗ
(1)¼ÓČė¹ĢĢåAgNO3£¬Ōņ[Ag£«]________(Ģī”°±ä“ó”±”¢”°±äŠ””±»ņ”°²»±ä”±£¬ĻĀĶ¬)£»
(2)¼ÓČėøü¶ąµÄAgBr¹ĢĢ壬Ōņ[Ag£«]________£»
(3)¼ÓČėAgCl¹ĢĢ壬Ōņ[Br£]________£¬[Ag£«]________£»
(4)¼ÓČėNa2S¹ĢĢ壬Ōņ[Br£]________£¬[Ag£«]________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠÓŠ¹ŲAgCl³ĮµķµÄČܽāĘ½ŗāĖµ·ØÕżČ·µÄŹĒ(””””)
A£®AgCl³ĮµķÉś³ÉŗĶ³ĮµķČܽā“ļĘ½ŗāŗó²»ŌŁ½ųŠŠ
B£®AgClÄŃČÜÓŚĖ®£¬ČÜŅŗ֊ƻӊAg£«ŗĶCl£
C£®ÉżøßĪĀ¶Č£¬AgCl³ĮµķµÄČܽā¶ČŌö“ó
D£®ĻņAgCl³ĮµķÖŠ¼ÓČėNaCl¹ĢĢ壬AgCl³ĮµķµÄČܽā¶Č²»±ä
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŌŚ50 mL 0.2 mol·L£1 CuSO4ČÜŅŗÖŠ²åČėĮ½øöµē¼«£¬Ķصēµē½ā(²»æ¼ĀĒĖ®·ÖÕō·¢)”£Ōņ£ŗ
(1)ČōĮ½¼«¾łĪŖĶʬ£¬ŹŌĖµĆ÷µē½ā¹ż³ĢÖŠCuSO4ČÜŅŗµÄÅضČ________(Ģī”°Ōö“ó”±”¢”°¼õŠ””±»ņ”°²»±ä”±)”£
(2)ČōŃō¼«ĪŖ“æŠæ£¬Ņõ¼«ĪŖĶʬ£¬Ńō¼«·“Ó¦Ź½ŹĒ________________________________________________________________________
________________________________________________________________________ӣ
(3)ČōŃō¼«ĪŖ“æŠæ£¬Ņõ¼«ĪŖĶʬ£¬Čē²»æ¼ĀĒH£«ŌŚŅõ¼«ÉĻ·Åµē£¬µ±µēĀ·ÖŠÓŠ0.04 mol e£ĶعżŹ±£¬Ņõ¼«ŌöÖŲ________g£¬Ņõ¼«ÉĻµÄµē¼«·“Ó¦Ź½ŹĒ________________________________________________________________________
________________________________________________________________________ӣ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŹŅĪĀŹ±£¬ĻĀĮŠČÜŅŗÖŠĪ¢Į£µÄÅØ¶Č¹ŲĻµÕżČ·µÄŹĒ””””)”£
A.0.1 mol”¤L£1 pH£½4µÄNaHSO3ČÜŅŗÖŠ£ŗcHSO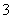 )>cSO
)>cSO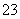 )>cH2SO3)
)>cH2SO3)
B.µČĢå»ż”¢µČĪļÖŹµÄĮæÅØ¶ČµÄNaFČÜŅŗÓėHFČÜŅŗ»ģŗĻ£ŗcNa£«)£½cF£)£«cHF)
C.ŌŚNaHAČÜŅŗÖŠŅ»¶ØÓŠ£ŗcNa£«)£«cH£«)£½cHA£)£«cOH£)£«cA2£)
D.cNH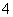 )ĻąµČµÄNH4)2CO3”¢NH4)2SO4ŗĶNH4)2FeSO4)2Čż·ŻČÜŅŗÖŠ£¬ČÜÖŹµÄĪļÖŹµÄĮæÅضČŅĄ“ĪŌö“ó
)ĻąµČµÄNH4)2CO3”¢NH4)2SO4ŗĶNH4)2FeSO4)2Čż·ŻČÜŅŗÖŠ£¬ČÜÖŹµÄĪļÖŹµÄĮæÅضČŅĄ“ĪŌö“ó
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com