
FeCl3������ӡˢ��·ͭ�帯ʴ��������ֹѪ����
��1����Ҫ�ܽ��·����3.2g��ͭ����������Ҫ1 mol��L��1 FeCl3��Һ�����Ϊ mL��
��2�����鸯ʴ��·ͭ������Һ���Ƿ����Fe3+���Լ��� ��
��3����ʴ��·ͭ������Һ��ͭԪ�غ����IJⶨ��
ȡ20.00mL��ʴ��·ͭ������Һ�ڵ���ƿ�У��ȼ�����NaF�������ķ�ӦΪFe3++6F����[FeF6]3�������ټ�������10%KI��Һ��ҡ�ȡ����ϵ���ƿƿ�������ڰ���5min����ַ�Ӧ����CuI�������ɣ����Ӽ��ε�����Һ����0.1000 mol��L?1Na2S2O3����Һ�ζ����յ�ʱ��������20.00mL��Һ���ⶨ�������й����ʵ�ת����ϵ���£�
���ⶨ�����е���ƿ�������ڰ���5min���ᵼ�²ⶨ��� �����ƫ�ߡ�����ƫ�͡�������Ӱ�족����
����ø�ʴҺ��ͭԪ�صĺ�������g��L?1��ʾ����д��������̡�
 ����˼ά�żӿ���ϵ�д�
����˼ά�żӿ���ϵ�д� �����Ծ�ϵ�д�
�����Ծ�ϵ�д� �ο�����������100��ϵ�д�
�ο�����������100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2017��������֥һ�и�����ѧ���¿�һ��ѧ�Ծ��������棩 ���ͣ��ƶ���
����ѧ��ѡ��5���л���ѧ������
����H�׳�ũ���ᣬ���Ʊ���Ч�ϼ��ȶ��־�ϸ��ѧƷ����Ҫԭ�ϣ��ɾ����з�Ӧ·�ߵõ���
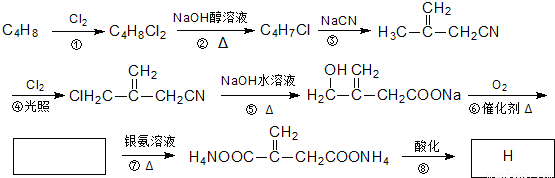
�ش��������⣺
��1��C4H8�����ƣ�ϵͳ��������____________��C4H7Cl�к��еĹ����ŵ�������____________��
��2����Ӧ�Ļ�ѧ����ʽΪ______________���䷴Ӧ����Ϊ_____________��?�ķ�Ӧ����____________��
��3���¿����ж���ͬ���칹�壬�������¿�������ͬ���Һ˴Ź���������5������л���Ľṹ��ʽΪ______________________��
��4�����й���H��˵����ȷ����_________������ĸ����
a����ʹ����KMnO4��Һ�����CCl4��Һ��ɫ
b������Na2CO3��Ӧ��������HBr��Ӧ
c����������Cu��OH��2��Ӧ
d��1 mol H��ȫȼ������5 mol O2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017��ӱ�������ѧ���������۲�������ѧ�Ծ��������棩 ���ͣ�ѡ����
��27.2gCu��Cu2O�Ļ�����м���ijŨ�ȵ�ϡ����0.5L������������ȫ��Ӧ������NO�� Cu��NO3��2����������Һ�м���l.0mol/L��NaOH��Һ1.0L����ʱ��Һ�����ԣ�������������ȫ��������������Ϊ39.2g�������й�˵������ȷ����
A��Cu��Cu2O�����ʵ���֮��Ϊ2:1
B����������ʵ���Ũ��Ϊ2.6mol/L
C��������NO�ڱ�״���µ����Ϊ4.48L
D��Cu��Cu2O�����ᷴӦ��ʣ��HNO3Ϊ0.2mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�콭��ʡ�γ��и�����ѧ�����л�ѧ�Ծ��������棩 ���ͣ�ѡ����
�����йذ�����ε�˵������ȷ����
A��NH3�����������
B������ʪ��ĺ�ɫʯ����ֽ���鰱��
C��������ζ���ˮ������Һ������ʱ��c��NH4+�� = c��Cl����
D������ʱ��0.1mol��L��1NH4Cl��Һ��ˮϡ�ͣ�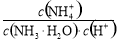 ��ֵ����
��ֵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�콭��ʡ�γ��и�����ѧ�����л�ѧ�Ծ��������棩 ���ͣ�ѡ����
���Ľ조�̷��ᡱ�����ǡ���ɫ��չ����������á���������Ϊ��������һ��ּ����
A����߷��ܡ�̫���ܵȿ����������Դ��ʹ�ñ���
B���ƹ�CO2�����ü���������ϳ��м�ֵ�Ļ�ѧƷ
C�����ù�ҵ��ˮ���ũ������ˮ��Դ��������
D���з�ú̿�Ľྻ����Ч���ü�����������̬����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�콭��ʡ���ݡ���Ǩ�����Ƹۡ��������и���11��ģ�⻯ѧ���������棩 ���ͣ�ѡ����
��������ʵ��������������õ��Ľ�����ȷ���ǣ� ��
ѡ�� | ʵ����������� | ���� |
A | ��1mL���������ֱ����6mLͬŨ�ȵ�NaOH��Һ��ϡH2SO4�У�ˮԡ������ͬʱ�����Һ��������ȫ��ʧ���������� | ˵������NaOH��Һ�е�ˮ��̶ȴ��� H2SO4��Һ�� |
B | ��10mL0.2mol��L?1NaOH��Һ�е���2��0.1 mol��L?1MgCl2��Һ��������ɫ�������ٵμ�2��0.1 mol��L?1FeCl3��Һ�����ɺ��ɫ���� | Ksp[Mg��OH��2]>Ksp[Fe��OH��3] |
C | �����£�ʵ���ã�0.1mol��L��1Na2CO3��Һ��pHԼΪ11.6��0.1mol��L��1 NaHCO3��Һ��pHԼΪ9.7 | CO32 |
D | ��3mLϡ����ֱ����������Zn����Zn�۷�Ӧ��Zn�۲�����������ʿ� | Zn�۵Ļ����Դ���Zn�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�콭��ʡ���ݡ���Ǩ�����Ƹۡ��������и���11��ģ�⻯ѧ���������棩 ���ͣ�ѡ����
����ָ����Ӧ�����ӷ���ʽ��ȷ���ǣ� ��
A����ϡ�� ��ϴ���Թ��ڱڵ�������Ag+2H��+NO3����Ag��+NO��+H2O
��ϴ���Թ��ڱڵ�������Ag+2H��+NO3����Ag��+NO��+H2O
B��̼��Ʒ�ĩ���������Һ�У�CaCO3+2H+��Ca2++CO2��+H2O
C��Ca��OH��2��Һ�����NaHCO3��Һ��Ӧ��HCO3��+Ca2��+OH����CaCO3��+H2O
D����ⱥ��NaCl��Һ��2Cl����2H2O 2OH����H2����Cl2��
2OH����H2����Cl2��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ������2017�������ѧ�ڵ������¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
���з�Ӧ�Ⱥ�˳���ж���ȷ����
A���ں������ʵ�����FeBr2��FeI2����Һ�л���ͨ��������I����Br����Fe2+
B���ں������ʵ�����NaCl��NaBr����Һ�м�����������Һ��Cl-��Br-
C���ں������ʵ�����Fe3+��Cu2+����Һ�м������ۣ�Cu2+��Fe3+
D���ں������ʵ�����H+��Al3+ ����Һ�У���μ���NaOH��Һ��H+��Al3+��Al��OH��3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����������и����ϵڶ���������⻯ѧ�Ծ��������棩 ���ͣ�ѡ����
ȡngij���������� ����ȫȼ�գ��������������Ĺ������ƹ�����ȫ��Ӧ����Ӧ����������ǡ��Ҳ������ng�������������������������������A��H2��CO�Ļ����B��C2H2O2C��C3H6O3D��C6H12O5
����ȫȼ�գ��������������Ĺ������ƹ�����ȫ��Ӧ����Ӧ����������ǡ��Ҳ������ng�������������������������������A��H2��CO�Ļ����B��C2H2O2C��C3H6O3D��C6H12O5
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com