
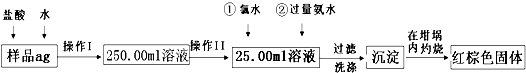
| V(��)��c(��) |
| V(����) |
| V(��)��c(��) |
| V(����) |
| 250mL |
| 25mL |
| 0.8m(V2-V1)g |
| Wg |
| 80m(V2-V1) |
| W |
| 80m(V2-V1) |
| W |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ����
���� ��Ӧѡ��
��Ӧѡ�� ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
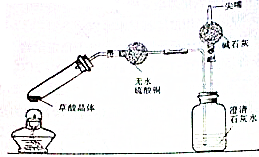 �����ϱ������Ҷ��ᣨHOOC-COOH���ɼ�дΪH2C2O4���׳Ʋ��ᣬ��һ�ְ�ɫ���壬������ˮ�����۵�Ϊ101.5�棬��157������������β�����ˮ��ijУ��ѧ�о���ѧϰС��Ϊ̽���������ȷֽ�IJ����ͬѧ�����ͼ��ʾ��װ�ã�װ�����Թܿ�������б����ԭ����
�����ϱ������Ҷ��ᣨHOOC-COOH���ɼ�дΪH2C2O4���׳Ʋ��ᣬ��һ�ְ�ɫ���壬������ˮ�����۵�Ϊ101.5�棬��157������������β�����ˮ��ijУ��ѧ�о���ѧϰС��Ϊ̽���������ȷֽ�IJ����ͬѧ�����ͼ��ʾ��װ�ã�װ�����Թܿ�������б����ԭ�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��1mol���ʯ�к���C-C������ĿΪ4NA |
| B��14��N2�к��еĦм���ĿΪNA |
| C��25�棬pH=13��NaOH��Һ�к���OH-����ĿΪ0.1NA |
| D��1L0.1mol/LAl2��SO4��3��Һ�к��е�Al3+����Ϊ0.2NA |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com