
(2)Ä³Ń§ÉśÅŠ¶ĻSO2ŗĶNa2O2·“Ó¦ÄÜÉś³ÉĮņĖįÄĘ£¬ÄćČĻĪŖĖūµÄÅŠ¶ĻŗĻĄķĀš£æ_________(Ģī”°ŗĻĄķ”±»ņ”°²»ŗĻĄķ”±)”£¼ņŅŖĖµĆ÷ĄķÓÉ____________________”£
(3)øĆĶ¬Ń§ĪŽ·ØČ·¶Ø·“Ó¦ÖŠŹĒ·ńÓŠŃõĘųÉś³É£¬ÄāÓĆČēĶ¼ĖłŹ¾×°ÖĆ½ųŠŠŹµŃ锣

×°ÖĆBµÄ×÷ÓĆŹĒ_____________________________________________”£
DµÄ×÷ÓĆŹĒ£ŗ_______________________________________________”£
(4)ĪŖČ·ČĻ·“Ó¦²śĪļ£¬ĒėĶź³ÉĻĀ±ķÖŠĖłĮŠŹµŃé
²Ł×÷ | ĻÖĻóÓė½įĀŪ |
¢ŁČ·ČĻŹĒ·ńÓŠŃõĘų²śÉśµÄ²Ł×÷ŹĒ£ŗ | ĻÖĻó£ŗ ½įĀŪ£ŗ |
¢ŚČ·ČĻŹĒ·ńÓŠĮņĖįÄĘ²śÉśµÄ²Ł×÷ŹĒ£ŗ | ĻÖĻó£ŗ ½įĀŪ |
½āĪö£ŗ±¾ĢāÖŠCO2ÓėNa2O2·“Ó¦ÕāŅ»Ö÷øÉÖŖŹ¶£¬ŃÓÉģĪŖSO2ÓėNa2O2µÄ·“Ó¦²śĪļµÄÅŠ¶ĻÓė¼ģŃ锣SO2ÓėCO2Ļą±Č½ĻĮ½Õß¹²Ķ¬Ö®“¦ŌŚÓŚ¾łĪŖĖįŠŌŃõ»ÆĪļ£¬²»Ķ¬Ö®“¦ŌŚÓŚSO2¾ßÓŠ½ĻĒæµÄ»¹ŌŠŌ”£ĖłŅŌSO2ÓėNa2O2µÄ·“Ó¦ÓėCO2ÓėNa2O2µÄ·“Ó¦¼ČÓŠĻąĖĘÖ®“¦ÓÖ“ęŌŚĒų±š”£ŌŚ·“Ó¦¹ż³ĢÖŠ£¬ÓÉÓŚNa2O2µÄĒæŃõ»ÆŠŌ£¬SŌŖĖŲµÄ»ÆŗĻ¼ŪÓ¦ÓÉ+4¼ŪÉżøßµ½+6¼Ū£¬·“Ó¦²śĪļÖŠæÉÄÜ»įÉś³ÉO2”£O2µÄ¼ģŃéæÉĄūÓĆĘäĢŲÕ÷¼ģŃé·Ø£ŗÄÜŹ¹“ų»šŠĒµÄľĢõø“Č¼”£
“š°ø£ŗ
(1)2Na2O2+2CO2![]() 2Na2CO3+O2ӟ
2Na2CO3+O2ӟ
(2)ŗĻĄķ ŅņĪŖNa2O2ÓŠĒæŃõ»ÆŠŌ£¬SO2ÓŠ»¹ŌŠŌ
(3)øÉŌļSO2 ĪüŹÕ¶ąÓąSO2·ĄÖ¹æÕĘų½ųČėCÖŠ£¬“Ó¶ųŹ¹Na2O2ÓėæÕĘųÖŠµÄĖ®·ÖŗĶCO2·“Ó¦
(4)
²Ł×÷ | ĻÖĻóÓė½įĀŪ |
¢Ł½«“ų»šŠĒľĢõ½Ó½üaæŚ | ľĢõø“Č¼£¬Ö¤Ć÷ÓŠO2Éś³É ľĢõ²»ø“Č¼£¬Ö¤Ć÷ĪŽO2Éś³É |
¢Ś½«CÖŠ¹ĢĢåČÜČėHClÖŠŌŁ¼ÓČėBaCl2ČÜŅŗ | ÓŠ³Įµķ²śÉś£¬Ö¤Ć÷ÓŠNa2SO4Éś³É ĪŽ³Įµķ²śÉś£¬Ö¤Ć÷ĪŽNa2SO4Éś³É |


 ĆæČÕ10·ÖÖÓæŚĖćŠÄĖćĖŁĖćĢģĢģĮ·ĻµĮŠ“š°ø
ĆæČÕ10·ÖÖÓæŚĖćŠÄĖćĖŁĖćĢģĢģĮ·ĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2013-2014ѧğÕć½Ź”ĮłŹŠĮłŠ£ĮŖĆĖøßæ¼Ä£Äāæ¼ŹŌĄķæĘ×ŪŗĻ»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
”°µĶĢ¼Ń»·”±ŅżĘšø÷¹śµÄø߶ČÖŲŹÓ£¬¶ųČēŗĪ½µµĶ“óĘųÖŠCO2µÄŗ¬Įæ¼°ÓŠŠ§µŲæŖ·¢ĄūÓĆCO2£¬ŅżĘšĮĖČ«ŹĄ½ēµÄĘÕ±éÖŲŹÓ”£ĖłŅŌ”°µĶĢ¼¾¼Ć”±Õż³ÉĪŖæĘѧ¼ŅŃŠ¾æµÄÖ÷ŅŖæĪĢā”£
£Ø1£©Š“³öCO2ÓėH2·“Ӧɜ³ÉCH4ŗĶH2OµÄČČ»Æѧ·½³ĢŹ½???????????????????? ”£
ŅŃÖŖ£ŗ? ¢Ł CO(g)+H2O(g) H2(g)+CO2(g)??? ¦¤H£½£41kJ”¤mol£1
H2(g)+CO2(g)??? ¦¤H£½£41kJ”¤mol£1
¢Ś C(s)+2H2(g) CH4(g)??????????? ¦¤H£½£73kJ”¤mol£1
CH4(g)??????????? ¦¤H£½£73kJ”¤mol£1
¢Ū 2CO(g) C(s)+CO2(g)???? ????? ¦¤H£½£171kJ”¤mol£1
C(s)+CO2(g)???? ????? ¦¤H£½£171kJ”¤mol£1
£Ø2£©½«Č¼Ćŗ·ĻĘųÖŠµÄCO2×Ŗ»ÆĪŖ¶ž¼×Ćѵķ“Ó¦ŌĄķĪŖ£ŗ2CO2(g) + 6H2(g)  CH3OCH3(g) + 3H2O(g)”£ŅŃÖŖŅ»¶ØĢõ¼žĻĀ£¬øĆ·“Ó¦ÖŠCO2µÄĘ½ŗā×Ŗ»ÆĀŹĖęĪĀ¶Č”¢Ķ¶ĮĻ±Č[n(H2) / n(CO2)]µÄ±ä»ÆĒśĻßČēĻĀ×óĶ¼£ŗ
CH3OCH3(g) + 3H2O(g)”£ŅŃÖŖŅ»¶ØĢõ¼žĻĀ£¬øĆ·“Ó¦ÖŠCO2µÄĘ½ŗā×Ŗ»ÆĀŹĖęĪĀ¶Č”¢Ķ¶ĮĻ±Č[n(H2) / n(CO2)]µÄ±ä»ÆĒśĻßČēĻĀ×óĶ¼£ŗ
¢ŁŌŚĘäĖūĢõ¼ž²»±äŹ±£¬ĒėŌŚÉĻĶ¼ÖŠ»³öĘ½ŗāŹ±CH3OCH3µÄĢå»ż·ÖŹżĖęĶ¶ĮĻ±Č[n(H2) / n(CO2)]±ä»ÆµÄĒśĻßĶ¼”£

¢ŚÄ³ĪĀ¶ČĻĀ£¬½«2.0molCO2(g)ŗĶ6.0molH2(g)³äČėČŻ»żĪŖ2LµÄĆܱÕČŻĘ÷ÖŠ£¬·“Ó¦µ½“ļĘ½ŗāŹ±£¬øıäŃ¹ĒæŗĶĪĀ¶Č£¬Ę½ŗāĢåĻµÖŠCH3OCH3(g)µÄĪļÖŹµÄĮæ·ÖŹż±ä»ÆĒéæöČēĶ¼ĖłŹ¾£¬¹ŲÓŚĪĀ¶ČŗĶŃ¹ĒæµÄ¹ŲĻµÅŠ¶ĻÕżČ·µÄŹĒ??? ? ??? ?? £»

A. P3£¾P2£¬T3£¾T2???????? B. P1£¾P3£¬T1£¾T3??? C. P2£¾P4£¬T4£¾T2???????? D. P1£¾P4£¬T2£¾T3
¢ŪŌŚŗćČŻĆܱÕČŻĘ÷Ąļ°“Ģå»ż±ČĪŖ1:3³äČė¶žŃõ»ÆĢ¼ŗĶĒā Ęų£¬Ņ»¶ØĢõ¼žĻĀ·“Ó¦“ļµ½Ę½ŗāדĢ¬”£µ±øı䷓ӦµÄijŅ»øöĢõ¼žŗó£¬ĻĀĮŠ±ä»ÆÄÜĖµĆ÷Ę½ŗāŅ»¶ØĻņÄę·“Ó¦·½ĻņŅĘ¶ÆµÄŹĒ ????? £»
A. Õż·“Ó¦ĖŁĀŹĻČŌö“óŗó¼õŠ”
B. Äę·“Ó¦ĖŁĀŹĻČŌö“óŗó¼õŠ”
C. »ÆŃ§Ę½ŗā³£ŹżKÖµŌö“ó
D. ·“Ó¦ĪļµÄĢå»ż°Ł·Öŗ¬ĮæŌö“ó
E. »ģŗĻĘųĢåµÄĆÜ¶Č¼õŠ”
F. ĒāĘųµÄ×Ŗ»ÆĀŹ¼õŠ”
£Ø3£©×ī½üæĘѧ¼ŅŌŁ“ĪĢį³ö”°ĀĢÉ«»Æѧ”±¹¹Ļė£ŗ°ŃæÕĘų“µČėĢ¼Ėį¼ŲČÜŅŗ£¬Č»ŗóŌŁ°ŃCO2“ÓČÜŅŗÖŠĢįČ”³öĄ“£¬¾»Æѧ·“Ó¦ŗóŹ¹æÕĘųÖŠµÄCO2×Ŗ±äĪŖæÉŌŁÉśČ¼ĮĻ¼×“¼”£¼×“¼æÉÖĘ×÷Č¼ĮĻµē³Ų£¬Š“³öŅŌĻ”ĮņĖįĪŖµē½āÖŹ¼×“¼Č¼ĮĻµē³Ųøŗ¼«·“Ó¦Ź½__?????????????????????????? ?? ”£ŅŌ“ĖČ¼ĮĻµē³Ų×÷ĪŖĶā½ÓµēŌ“°“Ķ¼ĖłŹ¾µē½āĮņĖįĶČÜŅŗ£¬Čē¹ūĘšŹ¼Ź±Ź¢ÓŠ1000mL pH£½5µÄĮņĖįĶČÜŅŗ£Ø25”ę£¬CuSO4×ćĮ棩£¬Ņ»¶ĪŹ±¼äŗóČÜŅŗµÄpH±äĪŖ1£¬“ĖŹ±æɹŪ²ģµ½µÄĻÖĻóŹĒ????????????????????? £»ČōŅŖŹ¹ČÜŅŗ»Öø“µ½ĘšŹ¼ÅØ¶Č£ØĪĀ¶Č²»±ä£¬ŗöĀŌČÜŅŗĢå»żµÄ±ä»Æ£©£¬æÉĻņČÜŅŗÖŠ¼ÓČė?????? £ØĢīĪļÖŹĆū³Ę£©£¬ĘäÖŹĮæŌ¼ĪŖ??? g”£

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012-2013Ń§ÄźÉ½¶«Ź”×Ķ²©ŹŠøßČżø“Ļ°½×¶ĪŠŌ¼ģ²ā£Ø¶žÄ££©ĄķæĘ×ŪŗĻ»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
(1)Š“³öCO2+Ąė×Ó»łĢ¬µÄ¼Ūµē×ÓÅŲ¼Ź½£ŗ__________________________________”£
(2)SO32-µÄæռ乹ŠĶŹĒ£ŗ___________________________________________”£
(3)OCN-ÓėCO2ŹĒµČµē×ÓĢ壬ŌņOCN-ÖŠCŌ×ÓµÄŌӻƷ½Ź½ŹĒ£ŗ_______________”£
(4)Įł·½µŖ»ÆÅš(BN)¾§Ģå¾ßÓŠŗÜøßµÄČŪµć£¬BŌ×ÓŗĶNŌ×Ó¾łĪŖsp2ŌӻƔ£øĆ¾§ĢåÖŠ“ęŌŚµÄ×÷ÓĆĮ¦ÓŠ£ŗ__________________”£
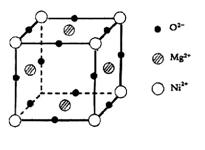
(5)ŌŖĖŲO”¢Mg”¢NiæÉŠĪ³ÉŅ»ÖÖ¾§Ģ壬Ę侧°ūČēĶ¼ĖłŹ¾”£ŌŚ¾§ĢåÖŠ£¬ĆæøöNi2+Ąė×ÓÓė_____________øöMg2+Ąė×ÓÅäĪ»”£øĆ¾§ĢåµÄ»ÆѧŹ½ŹĒ____________”£

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗÄ£ÄāĢā ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾»Æѧ”Ŗ”ŖĪļÖŹ½į¹¹ÓėŠŌÖŹ”æ
(1)Š“³öCO2+Ąė×Ó»łĢ¬µÄ¼Ūµē×ÓÅŲ¼Ź½£ŗ__________________________________”£
(2)SO32-µÄæռ乹ŠĶŹĒ£ŗ___________________________________________”£
(3)OCN-ÓėCO2ŹĒµČµē×ÓĢ壬ŌņOCN-ÖŠCŌ×ÓµÄŌӻƷ½Ź½ŹĒ£ŗ_______________”£
 (4)Įł·½µŖ»ÆÅš(BN)¾§Ģå¾ßÓŠŗÜøßµÄČŪµć£¬BŌ×ÓŗĶNŌ×Ó¾łĪŖsp2ŌӻƔ£øĆ¾§ĢåÖŠ“ęŌŚµÄ×÷ÓĆĮ¦ÓŠ£ŗ__________________”£
(4)Įł·½µŖ»ÆÅš(BN)¾§Ģå¾ßÓŠŗÜøßµÄČŪµć£¬BŌ×ÓŗĶNŌ×Ó¾łĪŖsp2ŌӻƔ£øĆ¾§ĢåÖŠ“ęŌŚµÄ×÷ÓĆĮ¦ÓŠ£ŗ__________________”£
(5)ŌŖĖŲO”¢Mg”¢NiæÉŠĪ³ÉŅ»ÖÖ¾§Ģ壬Ę侧°ūČēĶ¼ĖłŹ¾”£ŌŚ¾§ĢåÖŠ£¬ĆæøöNi2+Ąė×ÓÓė_____________øöMg2+Ąė×ÓÅäĪ»”£øĆ¾§ĢåµÄ»ÆѧŹ½ŹĒ____________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
(1)ŌŚ101 kPaŹ±£¬COŌŚ1.0 mol O2ÖŠĶźČ«Č¼ÉÕ£¬Éś³É2.0 mol CO2£¬·Å³ö566.0 kJµÄČČĮ棬 Ōņ“Ė·“Ó¦µÄČČ»Æѧ·½³Ģ£ŗ”””””””””””””””””””””””””””””””” ;
ÓÖŅŃÖŖ£ŗ2H2(g)£«O2(g)=2H2O(g)£»¦¤H£½£483.6 kJ£Æmol£¬ĒėŠ“³öCO2ÓėH2·“Ӧɜ³ÉCOŗĶĖ®ÕōĘųµÄČČ»Æѧ·½³Ģ£ŗ”””””””””””””””””””””””””””””””””””””””””””””” ”£
(2) ²šæŖ1molH-H¼ü”¢1molN-H¼ü”¢1molN”ŌN¼ü·Ö±šŠčŅŖÄÜĮæŹĒ436KJ”¢391KJ”¢946KJ£¬ČōÓŠ1mol N2ÓėH2Ē”ŗĆĶźČ«·“Ӧɜ³ÉNH3£¬Ōņ·“Ó¦µÄ¦¤H£½”””””””””””””” ”£
(3) ijĪĀ¶ČŹ±£¬ŌŚ2 LČŻĘ÷ÖŠX”¢Y”¢ZČżÖÖĪļÖŹµÄĮæĖę Ź±¼äµÄ±ä»ÆĒśĻßČēÓŅĶ¼ĖłŹ¾”£ÓÉĶ¼ÖŠŹż¾Ż·ÖĪö£¬øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ£ŗ”””””””””””””””””””””” ”£
(4) Ņ»¶ØĪĀ¶ČĻĀµÄĆܱÕČŻĘ÷ÖŠ“ęŌŚČēĻĀ·“Ó¦£ŗ
2SO2(g)£«O2(g) ![]() 2SO3(g)£¬ŅŃÖŖcŹ¼(SO2)£½0.4mol”¤L£1£¬cŹ¼(O2)£½1mol”¤L£1£¬¾²ā¶ØøĆ·“Ó¦ŌŚøĆĪĀ¶ČĻĀµÄĘ½ŗā³£ŹżK=19£¬ŹŌÅŠ¶Ļ£ŗ
2SO3(g)£¬ŅŃÖŖcŹ¼(SO2)£½0.4mol”¤L£1£¬cŹ¼(O2)£½1mol”¤L£1£¬¾²ā¶ØøĆ·“Ó¦ŌŚøĆĪĀ¶ČĻĀµÄĘ½ŗā³£ŹżK=19£¬ŹŌÅŠ¶Ļ£ŗ
µ±SO2×Ŗ»ÆĀŹĪŖ50%Ź±£¬øĆ·“Ó¦”””””””” £ØĢī”°ŹĒ”±»ņ”°·ń”±£©“ļµ½Ę½ŗāדĢ¬£¬ČōĪ““ļµ½£¬ŌņĻņ”””””””””” £ØĢī”°Õż”±»ņ”°Äę”±£¬ ČōŅŃ“ļµ½Ę½ŗā“ĖæÕæɲ»Ģī£©·½Ļņ½ųŠŠ”£

²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com