
(8��)���̷��е����ʺ����IJⶨ�������á����϶�����������ԭ���ǣ�ʳƷ������ʹ���һͬ����������ʹ�����ʷֽ⣬�ֽ�İ�����������������李�Ȼ������ʹ�����룬���������պ�����������������Һ�ζ�������������������Ի���ϵ������Ϊ�����ʺ�����ʵ��������裺
(1)��Ʒ������ȷ��ȡһ�����Ĺ�����Ʒ�̷ۣ��������Ŀ�����ƿ�У�����һϵ�еĴ���������ȴ������һ�����������ƿ�С�
(2)NH3����������գ����Ƶõ���Һ��ȡһ��������ͨ������װ�ã�����һϵ�еķ�Ӧ��ʹ���������泥��پ�������������Ϊ����״̬�����백���������ա�
(3)���ĵζ����ñ�������Һ�ζ������ɵ�����泥������ĵ������Һ������ܵ�����������Ϊ�ֵ�������
�Իش��������⣺
(1)����Ʒ�Ĵ���������ʹ�õ�������ƿ�������������ƿ�Ƿ�©ˮ��
(2)�����ƹ����У����������������ʹ���Ƶ���Һ��Ũ��ƫ�� ��
A��������ƿ����Һת���Ƶ�����ƿ��ʱ��δϴ�ӿ�����ƿ
B������ʱ�����ӿ̶��� C������ʱ�����ӿ̶��� D����Һʱ��������Һ�彦��
 (3)����ȡ��Ʒ������Ϊ1.5g��������100��������Һ��ȡ���е�20mL������һϵ�д�����ʹNת��Ϊ�����Ȼ����0.1mol/L����ζ�������ȥ��������Ϊ23.0mL�������Ʒ��N�ĺ���Ϊ ��(��֪���ζ��������漰���ķ�Ӧ����ʽ��(NH4)2 B4O7 + 2HCl + 5H2O = 2NH4Cl + 4H3BO3)
(3)����ȡ��Ʒ������Ϊ1.5g��������100��������Һ��ȡ���е�20mL������һϵ�д�����ʹNת��Ϊ�����Ȼ����0.1mol/L����ζ�������ȥ��������Ϊ23.0mL�������Ʒ��N�ĺ���Ϊ ��(��֪���ζ��������漰���ķ�Ӧ����ʽ��(NH4)2 B4O7 + 2HCl + 5H2O = 2NH4Cl + 4H3BO3)
(4)һЩ������ũ���á����϶�������ֻ��Ԫ�صĺ������ó������ʵĺ��������ⷨ��ȱ��,�Ա�ţ�̼��ʱ�����ʵĺ������,����ţ�������������谷��C3N6H6������ʵ���϶�������ȱ�ݲ������ֲ���ֻҪ�����������ᴦ����Ʒ�������������õ������γɳ��������˺ֱ�ⶨ��������Һ�еĵ��������Ϳ���֪�������ʵ�����������ð�䵰���ʵĵ����������������谷�е��ĺ���Ϊ ��
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
���ڡ������̷ۡ����������ǵļ����ע����������Ҫ���̷��в����������谷�������谷��״Ϊ����ɫ���壬��ζ���ܶ�1.573�ˣ�����3 ��16�棩����ѹ���۵�Ϊ354�棨�ֽ⣩�����ټ��������������¶�Ϊ300�棻������ˮ����������ˮ���ѡ��������Ȼ�̼�������ڼ״������ᡢ���Ҷ����ȡ�
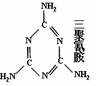
��1�������谷�Ľṹ��ʽ����ͼ��ʾ�Ļ�״��������谷�ķ���ʽΪ_________�����ʵĺ�����Ϊ �������谷Ϊ�����谷���ӼӾ۶��ɣ���֪�谷�����г�Hԭ���⣬C��N ԭ�ӵ���������8�����ӵĽṹ�����谷�ĵ���ʽΪ_______________�� �����谷����Է�������������������Ƚ� ����ɫ����ɫ�߿�����ζ����״Һ�塣��Է�������Ϊ123.11���۵�5.7�档�е�210.9�棩�������谷���۵�����ĸߣ���ԭ����______________________________��

��2�����й��������谷��˵���У���ȷ����________________������ţ���ѡ�۷֣���
A. �����谷��һ�ֺ������ߵİ�ɫ�ᾧ����ζ�����Բ����̷ۺ��ױ�����
B. �����谷����������ԭ��һ����ͬһ��ƽ����
C. �����谷��������ˮ�����ڷ��Ӿ���
D. �����谷�������ԣ��������ᷴӦ
E. ���������谷�����ʳ��һ�㶼����������ɷŽ���¯ʹ�á�
��3������Ʒ�Ļ���ϵ��Ϊ6.38�������������ĺ���Ϊ1%�������ʵĺ�����Ϊ6.38%����������ͨ���ڵ͵��������̷��м��������谷������ߡ��̷��еĵ����ʺ�������������Ӥ������ʯ���ٶ��̷��е����ʺ���Ϊ16%��Ϊ�ϸ���������һ��������Ϊ500g�������ʺ���Ϊ0�ļ��̷��в�����________g�������谷�Ϳ�ʹ�̷ۡ���ꡱ��С�������1λ��Ч���֣���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
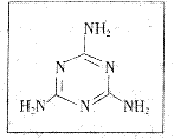
(17��)�ݷ����2008��9��11�ű��������ڸ���ȵر���൹Ӥ������ϵͳ��ʯ��������������뻼��ʳ����¹��Ӥ���䷽�̷ۺ��е������谷�йء���֪�������谷Ϊ��ɫ���壬��������
 ʳƷ���Ӽ������ʳƷ�е����ʵļ��ֵ���׳ơ�����������
ʳƷ���Ӽ������ʳƷ�е����ʵļ��ֵ���׳ơ�����������
����ʽΪ��ͼ��ʾ��
��ش��������⡣
(1)�����谷�ķ���ʽΪ ____________________��
(2)��֪�谷�����г�![]() ԭ���⣬����
ԭ���⣬����![]() ԭ�ӵ�����㶼����8���ӵĽṹ�����谷���ӵĵ���ʽΪ___________________��
ԭ�ӵ�����㶼����8���ӵĽṹ�����谷���ӵĵ���ʽΪ___________________��
(3)�����谷��ǿ���ǿ��������ˮ������ղ�������������![]() �����������������������β���е�
�����������������������β���е�![]() ����Ӧԭ��Ϊ��
����Ӧԭ��Ϊ��![]() ��
��
![]() ������˵����ȷ����___________(����ĸ���)��
������˵����ȷ����___________(����ĸ���)��
A��![]() ��
��![]() ��ͬϵ�� B��
��ͬϵ�� B��![]() ��һ��ǿ������
��һ��ǿ������
C�� ![]() �ڷ�Ӧ��ת�Ƶĵ���Ϊ4mol D����Ӧ��
�ڷ�Ӧ��ת�Ƶĵ���Ϊ4mol D����Ӧ��![]() �ǻ�ԭ��
�ǻ�ԭ��
��˳��ϩ�����������һ����Ҫ���л�����ԭ��,�㷺Ӧ����Ϳ��,���ᣬɱ�����������ˮ�������ȷ��档����������ʯ�Ͳ�ƷM�ϳ�˳��ϩ����������IJ��ֹ���
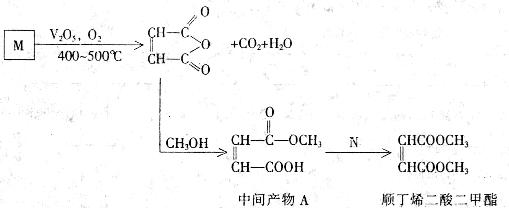
(1)��֪��M����Է�������Ϊ78�������ں�̼��Ϊ92.3��������C�DH����̼̼��������ͬ��������M�ķ���ʽΪ______________________��
(2)�����м����A���еĹ�����Ϊ_____________________(������)��
(3)д�����м����A�ϳ�˳��ϩ����������ķ���ʽ____________________________________________��
(4)�м����A��������![]() ��Ӧ�ɵ��л���
��Ӧ�ɵ��л���![]() ��
��
��д��B���ڶ�������˴Ź����������������շ壬������֮��Ϊ3:1�Ľṹ��ʽ______________��
��д��B���ڶ�������˴Ź����������������շ壬������֮��Ϊl:2:1�Ľṹ��ʽ______________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com