
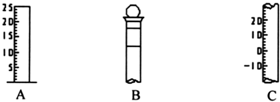
 ����ͼ��ʾΪ���������IJ��ֽṹ��
����ͼ��ʾΪ���������IJ��ֽṹ�� �������ʵ������ӻ���Һ�������Сʱ��ʵ���ܶ�ƫ��
�������ʵ������ӻ���Һ�������Сʱ��ʵ���ܶ�ƫ�� �������ʵ������ӻ���Һ�������Сʱ��ʵ���ܶ�ƫ���������жϣ�
�������ʵ������ӻ���Һ�������Сʱ��ʵ���ܶ�ƫ���������жϣ�

 ����ȫ���ִʾ��ƪ��ϵ�д�
����ȫ���ִʾ��ƪ��ϵ�д� �����߿����ϵ�д�
�����߿����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ����ͼ��ʾΪ���������IJ��ֽṹ��
����ͼ��ʾΪ���������IJ��ֽṹ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
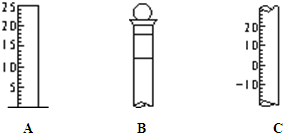 ��ͼ��ʾΪ���������IJ��ֽṹ��
��ͼ��ʾΪ���������IJ��ֽṹ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| 1 | 10 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
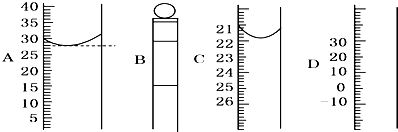
��4�֣���ͨ��״���£���ͬѧȡ1 mol H2O���ȵ�100��ʱ��Һ̬ˮ������Ϊˮ��������ͼ��ʾ�����ù������� �� �仯��
�ڱ���ѹǿ���������£�ˮ��������� ��
���������������������22.4L ��
����ͬѧ��H2��O2��ȼ�յ�ʵ�飬��ʵ��������� �� �仯�� �ڸñ仯�����У�һ��������ȵ��� �� ������ţ���
A����Ӧ�������Ŀ�������������Ŀ B����Ӧ��ԭ�������ʵ�����������ԭ�������ʵ���
C����Ӧ���������������������� D����Ӧ����������������
��6�֣���ͼ��ʾΪ���������IJ��ֽṹ��
����д���������������ƣ� A �� ��B �� ��C �� ��
������B�ϱ���� �� ������ţ���
������ ���¶� �ۿ̶��� ��Ũ�� ���ݻ�
�Ǽ�������B�Ƿ�©ˮ�ķ����� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012�����ʡ��һ��ѧ�����п��Ի�ѧ�Ծ� ���ͣ�ʵ����
��18�֣���.��ͼ��ʾΪ���������IJ��ֽṹ��
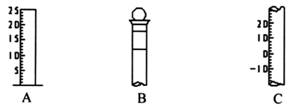
��1����д���������������ƣ�A ��B ��C ��
��2������B�ϱ���� ������ţ���
������ ���¶� �ۿ̶��� ��Ũ�� ���ݻ�
��3������Bʹ��ǰ���� ��
��.����98%��ŨH2SO4���ܶ�Ϊ1.84g/cm3��������480mL0.2mol��L��ϡH2SO4��
�йز���Ϊ���ټ�������Ũ�������� ����ȡһ�������Ũ���� ��ϡ�͡���ȴ ��ת�ơ�ϴ�� �ݶ��� ��ҡ��
�ش���������
��4��Ӧ��ȡ��Ũ��������� ��ʵ�������õIJ����������ձ�����������
��Ͳ����ͷ�ι���� ��
��5���ڢ۲���ϡ��Ũ����IJ�����
��6���������Ƶ�ϡH2SO4���вⶨ������ʵ��Ũ�ȴ���0.2mol��L���������������Щ��������������Ũ��ƫ����д��ĸ�� ��
A������Ͳ��ȡŨ����ʱ��������Ͳ�Ŀ̶�
B������ƿδ���T����������Һ
C��Ũ�������ձ���ϡ�ͺ�δ��ȴ������ת�Ƶ�����ƿ�У������ж���
D��������ƿת��ʱ��������Һ�彦��
E���ձ�δ����ϴ��
F��������ƿ�ж���ʱ��������ƿ�̶���
G�����ݺ�����ƿ������ҡ�ȣ����ú�Һ�治���̶��ߣ��ټ�ˮ���̶��ߡ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com