
”¾ĢāÄæ”æĶ¼ĪŖĻø°ūÄŚÄ³Š©ÖŲŅŖ»ÆŗĻĪļµÄŌŖĖŲ×é³É¼°Ļą»„¹ŲĻµ£¬a”¢b±ķŹ¾ÓŠ»śŠ”·Ö×ÓĪļÖŹ£¬A”¢B“ś±ķÓŠ»ś“ó·Ö×ÓĪļÖŹ(A±ķŹ¾DNA)”£ Ēė·ÖĪö»Ų“šĻĀĮŠĪŹĢā:

(1)Ķ¼ÖŠX“ś±ķµÄ»ÆѧŌŖĖŲŹĒ____£¬Y“ś±ķµÄÖ÷ŅŖ»ÆѧŌŖĖŲŹĒ____”£ ·Ö×ÓbµÄ½į¹¹ĶØŹ½ŹĒ______”£
(2)Ķ¼ÖŠAŹĒ¾ų“󶹏żÉśĪļĻø°ūÄŚÖü“ęŅÅ“«ŠÅĻ¢µÄĪļÖŹ£¬Ę仳±¾×é³Éµ„Ī»aŹĒÖø_______£¬ÓŠ____ÖÖ”£
(3)BÓŠ¶ąÖÖ¹¦ÄܵÄŌŅņæÉŅŌĪŖ________________________
(4)ij·Ö×ÓB¾ĆøĖ®½āŗóµĆµ½ČōøÉʬ¶Ī,ĘäÖŠŅ»øöʬ¶Ī½į¹¹ČēĶ¼ĖłŹ¾:
![]()
øĆʬ¶ĪµÄĆū³ĘŹĒ____£¬Čō½«øĆʬ¶ĪĖ®½ā£¬¶ĻĮŃµÄ²æĪ»Ćū³ĘĪŖ____________£¬ÄܲśÉś____ÖÖ°±»łĖį£¬ĘäĖ®½ā²śĪļµÄĻą¶Ō·Ö×ÓÖŹĮæÖ®ŗĶ±ČøĆĪļÖŹµÄĻą¶Ō·Ö×ÓÖŹĮæ¶ąĮĖ____”£
”¾“š°ø”æN.P N ![]() ĶŃŃõŗĖÜÕĖį 4 °±»łĖįµÄÖÖĄą”¢°±»łĖįµÄŹżÄ攢°±»łĖįµÄÅÅĮŠĖ³ŠņŅŌ¼°¶ąėÄĮ“µÄæÕ¼ä½į¹¹£Ø»ņÕß×īŗóŅ»øöĢīÅĢĒśÕŪµž·½Ź½£©²»Ņ»Ńł ĪåėÄ ėļü 4 72
ĶŃŃõŗĖÜÕĖį 4 °±»łĖįµÄÖÖĄą”¢°±»łĖįµÄŹżÄ攢°±»łĖįµÄÅÅĮŠĖ³ŠņŅŌ¼°¶ąėÄĮ“µÄæÕ¼ä½į¹¹£Ø»ņÕß×īŗóŅ»øöĢīÅĢĒśÕŪµž·½Ź½£©²»Ņ»Ńł ĪåėÄ ėļü 4 72
”¾½āĪö”æ
½ā“š±¾ĢāµÄ¹Ų¼üŹĒ¶Ō·Ö×ÓA”¢B”¢a”¢b¼°ŌŖĖŲX”¢YµÄŹ¶±š”£øł¾ŻĶ¼Ź¾æÉÖŖ£¬BŹĒµ°°×ÖŹ£ØŅņĪŖĶ¼ÖŠÓŠBµÄ¹¦ÄÜ£©£¬A¾ö¶ØB£¬ĖłŅŌAŹĒDNA”£µ°°×ÖŹÓÉC”¢H”¢O”¢NŌŖĖŲ¹¹³É£¬ÓŠŠ©ŗ¬ÓŠP”¢S”£»ł±¾µ„Ī»£ŗ°±»łĖį£¬Ō¼20ÖÖ£¬½į¹¹ĢŲµć£ŗĆæÖÖ°±»łĖį¶¼ÖĮÉŁŗ¬ÓŠŅ»øö°±»łŗĶŅ»øöōČ»ł£¬²¢ĒŅĖūĆĒ¶¼Į¬½įŌŚĶ¬Ņ»øöĢ¼Ō×ÓÉĻ”£
£Ø1£©øł¾ŻĶ¼Ź¾æÉÖŖ£¬BŹĒµ°°×ÖŹ£ØŅņĪŖĶ¼ÖŠÓŠBµÄ¹¦ÄÜ£©£¬A¾ö¶ØB£¬ĖłŅŌAŹĒDNA£¬ŌņX“ś±ķµÄ»ÆѧŌŖĖŲĪŖNŗĶP”£·Ö×ÓbŹĒ°±»łĖį£¬ŌņY“ś±ķµÄÖ÷ŅŖ»ÆѧŌŖĖŲŹĒN”£°±»łĖįµÄĶØŹ½ŹĒ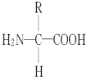 ”£
ӣ
£Ø2£©Ķ¼1ÖŠAŹĒDNA£¬ŹĒ¾ų“󶹏żÉśĪļµÄŅÅ“«ĪļÖŹ£¬¼“ŹĒĻø°ūÄŚÖü“ęŅÅ“«ŠÅĻ¢µÄĪļÖŹ£»Ę仳±¾×é³Éµ„Ī»aÖøµÄŹĒ4ÖÖĶŃŃõŗĖÜÕĖį”£
£Ø3£©B£Øµ°°×ÖŹ£©ÓŠ¶ąÖÖ¹¦ÄܵÄŌŅņŹĒŅņĪŖ×é³Éµ°°×ÖŹµÄ°±»łĖįµÄÖÖĄą”¢°±»łĖįµÄŹżÄ攢°±»łĖįµÄÅÅĮŠĖ³ŠņŅŌ¼°¶ąėÄĮ“µÄæÕ¼ä½į¹¹£Ø»ņÕß×īŗóŅ»øöĢīÅĢĒśÕŪµž·½Ź½£©²»Ņ»Ńł”£
£Ø4£©·ÖĪöĶ¼ŠĪæÉÖŖøĆʬ¶Īŗ¬ÓŠ4øöėļü£ØCO-NH-£©£¬ĖłŅŌøĆ»ÆŗĻĪļŹĒĪåėÄ”£Čō½«øĆʬ¶ĪĖ®½ā£¬¶ĻĮŃµÄ²æĪ»Ćū³ĘĪŖėļü£¬ĘäĖ®½āĻūŗÄ4·Ö×ÓĖ®£¬·Ö×ÓÖŹĮæÖ®ŗĶ±ČøĆĪļÖŹµÄ·Ö×ÓÖŹĮæ¶ąĮĖ18”Į4=72”£øł¾ŻR»łĶŵIJ»Ķ¬£ØÓŠ-CH2-C6H5”¢-CH3”¢-(CH2)4-NH2”¢-CH2-COOHĖÄÖÖR»łĶÅ£©æÉÖŖøĆ»ÆŗĻĪļÓÉ4ÖÖ°±»łĖįĶŃĖ®ĖõŗĻ¶ų³É”£



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æŹ®¾Å“ó±ØøęÖŠĢį³öŅŖ”°“ņÓ®Ą¶Ģģ±£ĪĄÕ½”±£¬ŅāĪ¶×ŶŌ“óĘųĪŪČ¾·ĄÖĪ±Č¹żČ„ŅŖĒóøüøß”£¶žŃõ»ÆĮņ”ŖæÕĘųÖŹ×Ó½»»»Ä¤Č¼ĮĻµē³ŲŹµĻÖĮĖÖĘĮņĖį”¢·¢µē”¢»·±£ČżĪ»Ņ»ĢåµÄ½įŗĻ£¬ŌĄķČēĶ¼ĖłŹ¾”£ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ

A. øƵē³Ų·ÅµēŹ±ÖŹ×Ó“ÓPt2µē¼«¾¹żÄŚµēĀ·Į÷µ½Pt1µē¼«
B. Pt1µē¼«ø½½ü·¢ÉśµÄ·“Ó¦ĪŖ£ŗSO2£«2H2O£2e££½H2SO4£«2H£«
C. Pt2µē¼«ø½½ü·¢ÉśµÄ·“Ó¦ĪŖO2£«4e££«2H2O£½4OH£
D. ĻąĶ¬Ģõ¼žĻĀ£¬·Åµē¹ż³ĢÖŠĻūŗĵÄSO2ŗĶO2µÄĢå»ż±ČĪŖ2”Ć1
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æÄ³Ń§ÉśÓĆ0.1mol/L KOHČÜŅŗµĪ¶ØĪ“ÖŖÅØ¶ČµÄŃĪĖį£¬Ęä²Ł×÷æÉ·Ö½āĪŖČēĻĀ¼ø²½£ŗ
£ØA£©ŅĘČ”20.00mL“ż²āµÄŃĪĖį×¢Čė½ą¾»µÄ׶ŠĪĘ棬²¢¼ÓČė2-3µĪ·ÓĢŖŹŌŅŗ
£ØB£©ÓƱź×¼ČÜŅŗČóĻ“µĪ¶Ø¹Ü2-3“Ī
£ØC£©°ŃŹ¢ÓŠ±ź×¼ČÜŅŗµÄ¼īŹ½µĪ¶Ø¹Ü¹Ģ¶ØŗĆ£¬µ÷½ŚŅŗĆęŹ¹µĪ¶Ø¹Ü¼ā×ģ³äĀśČÜŅŗ
£ØD£©Č”±ź×¼KOHČÜŅŗ×¢Čė¼īŹ½µĪ¶Ø¹ÜÖĮ0æĢ¶ČŅŌÉĻ2-3cm
£ØE£©µ÷½ŚŅŗĆęÖĮ0»ņ0æĢ¶ČŅŌĻĀ£¬¼ĒĻĀ¶ĮŹż
£ØF£©°Ń׶ŠĪĘæ·ÅŌŚµĪ¶Ø¹ÜµÄĻĀĆę£¬ÓƱź×¼KOHČÜŅŗµĪ¶ØÖĮÖÕµć£¬¼ĒĻĀµĪ¶Ø¹ÜŅŗĆęµÄæĢ¶Č
Ķź³ÉŅŌĻĀĢīæÕ£ŗ
£Ø1£©ÕżČ·²Ł×÷µÄĖ³ŠņŹĒ£ØÓĆŠņŗÅ×ÖÄøĢīŠ“£©______________________”£
£Ø2£©ÉĻŹö£ØB£©²Ł×÷µÄÄæµÄŹĒ___________________________________”£
£Ø3£©ÉĻŹö£ØA£©²Ł×÷Ö®Ē°£¬ČēĻČÓĆ“ż²āŅŗČóĻ“׶ŠĪĘ棬Ōņ¶Ō²ā¶Ø½į¹ūµÄÓ°ĻģŹĒ£ØĢīĘ«“ó”¢Ę«Š””¢²»±ä£¬£©______________”£
£Ø4£©ŹµŃéÖŠÓĆ×óŹÖæŲÖĘ_________£ØĢīŅĒĘ÷¼°²æĪ»£©£¬ŃŪ¾¦×¢ŹÓ_______£¬Ö±ÖĮµĪ¶ØÖÕµć”£ÅŠ¶Ļµ½“ļÖÕµćµÄĻÖĻóŹĒ_______________________________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĻĀĮŠøł¾Ż·“Ó¦ŌĄķÉč¼ĘµÄÓ¦ÓĆ£¬²»ÕżČ·µÄŹĒ
A. CO32£ + H2O ![]() HCO3£+ OH£ Čȵēæ¼īČÜŅŗĒåĻ“ÓĶĪŪ
HCO3£+ OH£ Čȵēæ¼īČÜŅŗĒåĻ“ÓĶĪŪ
B. Al3+ + 3H2O ![]() Al(OH)3£Ø½ŗĢ壩+ 3H+ Ć÷·Æ¾»Ė®
Al(OH)3£Ø½ŗĢ壩+ 3H+ Ć÷·Æ¾»Ė®
C. SnCl2 + H2O ![]() Sn(OH)Cl”ż + HCl ÅäÖĘĀČ»ÆŃĒĪżČÜŅŗŹ±¼ÓČėĒāŃõ»ÆÄĘ
Sn(OH)Cl”ż + HCl ÅäÖĘĀČ»ÆŃĒĪżČÜŅŗŹ±¼ÓČėĒāŃõ»ÆÄĘ
D. TiCl4+ £Øx+2£©H2O£Ø¹żĮ棩 ![]() TiO2”¤x H2O”ż + 4HCl ÓĆTiCl4ÖʱøTiO2
TiO2”¤x H2O”ż + 4HCl ÓĆTiCl4ÖʱøTiO2
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĻĀĮŠø÷×éĪļÖŹÖŠ£¬µŚŅ»ÖÖŹĒĖį£¬µŚ¶žÖÖŹĒ»ģŗĻĪļ£¬µŚČżÖÖŹĒ¼īµÄŹĒ£Ø £©
A.ĮņĖį”¢CuSO45H2O”¢æĮŠŌÄĘ
B.ĮņĖį”¢æÕĘų”¢“æ¼ī
C.Ńõ»ÆĢś”¢µØ·Æ”¢ŹģŹÆ»Ņ
D.ĻõĖį”¢Ź³ŃĪĖ®”¢ÉÕ¼ī
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æŅ»ÖÖÖ²ĪļŗĶŅ»ÖÖ²øČé¶ÆĪļĢåÄŚĻø°ūµÄijŠ©»ÆѧŌŖĖŲŗ¬Įæ(Õ¼Ļø°ūøÉÖŲµÄÖŹĮæ·ÖŹż)ČēĻĀ±ķĖłŹ¾£¬ĻĀĮŠÓŠ¹ŲŠšŹöÕżČ·µÄŹĒ

A. Ö²ĪļĻø°ūÖŠOŗ¬ĮæŗÜøßÓėÖ¬ÖŹŗ¬ĮæøßÓŠ¹Ų
B. ÕāĮ½ÖÖÉśĪļĢåÄŚĖłŗ¬µÄ»ÆѧŌŖĖŲµÄÖÖĄą²īŅģŗÜ“ó
C. µ°°×ÖŹÖŠµÄNÖ÷ŅŖ“ęŌŚÓŚėļüÖŠ
D. ¾Ż±ķĶĘ²āøĆÖ²ĪļŗĶ¶ÆĪļĢåÄŚ²»“ęŌŚĪ¢ĮæŌŖĖŲ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĪļÖŹÖ®¼ä·¢Éś»Æѧ·“Ó¦Ź±£¬Ņ»¶Ø·¢Éś±ä»ÆµÄŹĒ£Ø £©
A.Ō×ÓŗĖB.»Æѧ¼üC.ŃÕÉ«D.דĢ¬
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æÓŠŅ»Ęæ³ĪĒåµÄČÜŅŗ£¬ĘäÖŠæÉÄÜŗ¬ÓŠNH4£«”¢K£«”¢Ba2£«”¢Al3£«”¢Fe3£«”¢I£”¢NO3£”¢CO32£”¢SO42£”¢AlO2£”£Č”øĆČÜŅŗ½ųŠŠŅŌĻĀŹµŃé£ŗ
¢ŁÓĆpHŹŌÖ½¼ģ²ā£¬ČÜŅŗ³ŹĒæĖįŠŌ£»
¢ŚČ”ČÜŅŗŹŹĮ棬¼ÓČėÉŁĮæCCl4ŗĶŹżµĪŠĀÖĘĀČĖ®£¬Õńµ“£¬CCl4²ć³Ź×ĻŗģÉ«£»
¢ŪĮķČ”ČÜŅŗŹŹĮ棬֚µĪ¼ÓČėNaOHČÜŅŗ£»
a£®ČÜŅŗ“ÓĖįŠŌ±äĪŖÖŠŠŌ
b£®ČÜŅŗÖš½„²śÉś³Įµķ
c£®³ĮµķĶźČ«Čܽā
d£®×īŗó¼ÓČČČÜŅŗ£¬ÓŠĘųĢå·Å³ö£¬øĆĘųĢåÄÜŹ¹ŹŖČóµÄŗģÉ«ŹÆČļŹŌÖ½±äĄ¶
¢ÜČ”ŹŹĮæ¢ŪµĆµ½µÄ¼īŠŌČÜŅŗ£¬¼ÓČėNa2CO3ČÜŅŗ£¬ÓŠ°×É«³ĮµķÉś³É”£
øł¾ŻÉĻŹöŹµŃéĻÖĻ󣬻Ų“šĻĀĮŠĪŹĢā”£
£Ø1£©ÓÉ¢ŁæÉŅŌÅųż__________µÄ“ęŌŚ”£
£Ø2£©ÓÉ¢ŚæÉŅŌÖ¤Ć÷__________µÄ“ęŌŚ£»Ķ¬Ź±Åųż______µÄ“ęŌŚ£»ĄķÓÉŹĒ_______________”£
£Ø3£©ÓÉ¢ŪæÉŅŌÖ¤Ć÷________µÄ“ęŌŚ£»Š“³öc”¢dĖłÉę¼°µÄ»Æѧ·½³ĢŹ½£¬ŹĒĄė×Ó·“Ó¦µÄÓĆĄė×Ó·½³ĢŹ½±ķŹ¾£ŗ c___________________________________________________ £»d___________________________________________________ ”£
£Ø4£©ÓÉ¢ÜæÉŅŌÅųż__________µÄ“ęŌŚ£¬Ķ¬Ź±Ö¤Ć÷________µÄ“ęŌŚ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æŗ£ŃóÖ²ĪļČēŗ£“ų”¢ŗ£ŌåÖŠŗ¬ÓŠ·įø»µÄµāŌŖĖŲ£¬µāŌŖĖŲŅŌµāĄė×ӵĊĪŹ½“ęŌŚ”£ŹµŃéŹŅ“Óŗ£ŌåÖŠĢįČ”µāµÄĮ÷³ĢČēĻĀ£ŗ
ŗ£Ōå![]() ŗ£Ōå»Ņ
ŗ£Ōå»Ņ![]() ŗ£Ōå»ŅŠü×ĒŅŗ
ŗ£Ōå»ŅŠü×ĒŅŗ![]()
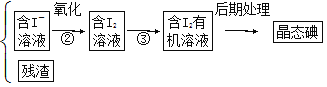
£Ø1£©Š“³öĢįČ”µāµÄ¹ż³ĢÖŠÓŠ¹ŲŹµŃé²Ł×÷µÄĆū³Ę£ŗ¢Ł______£»¢Ū________”£
£Ø2£©ĢįČ”µāµÄ¹ż³ĢÖŠæɹ©Ń”ŌńµÄÓŠ»śČܼĮŹĒ£Ø____£©
A£®ĘūÓĶ”¢¾Ę¾« B£®ĖÄĀČ»ÆĢ¼”¢ĘūÓĶ C£®“×Ėį”¢¾Ę¾«
£Ø3£©ĪŖĶź³ÉŅŌÉĻ¢Ł”¢¢ŪĮ½²½²Ł×÷£¬ŹµŃéŹŅĄļÓŠÉÕ±”¢²£Į§°ō”¢Ģś¼ÜĢØ”¢ÉÕĘ攢µ¼¹Ü”¢¾Ę¾«µĘ£¬ÉŠČ±ÉŁµÄ²£Į§ŅĒĘ÷ŹĒ____________________________________”£
£Ø4£©“Óŗ¬µāµÄÓŠ»śČܼĮÖŠĢįČ”µāŗĶ»ŲŹÕÓŠ»śČܼĮ£¬»¹ŠčŅŖ¾¹żÕōĮó”£Öø³öĻĀĶ¼ĖłŹ¾µÄŹµŃé×°ÖĆÖŠµÄ“ķĪóÖ®“¦£ŗ¢Ł______£¬¢Ś________£¬¢Ū________£¬¢Ü________”£

£Ø5£©ĪŖ±ćÓŚæŲÖĘÕōĮ󏱵ÄĪĀ¶Č£¬²Ł×÷Ź±Ź¹ÓĆĖ®Ō”¼ÓČČ£¬×īŗó¾§ĢåµāŌŚ________Ąļ¾Ū¼Æ”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com