
ij»ÆѧĢ½¾æŠ”×éÓū¶ŌSO2µÄ»ÆѧŠŌÖŹ½ųŠŠČēĻĀĢ½¾æ,ĒėÄć°ļÖśĖūĶź³ÉŹµŃé±Øøę”£
| ĪļÖŹ | Ąą±š | »ÆѧŠŌ ÖŹŌ¤²ā | ŹµŃéŃéÖ¤ | ||
| ŹµŃé²Ł×÷ | ŹµŃéĻÖĻó | ŹµÖŹ(ÓĆĄė×Ó ·½³ĢŹ½±ķŹ¾) | |||
| ¶žŃõ »ÆĮņ | ĖįŠŌ Ńõ»ÆĪļ | ÓėĖ® ·“Ó¦ | ½«Ź¢ĀśSO2ĘųĢåµÄŹŌ¹Üµ¹Į¢ŌŚĖ®ÖŠ,²¢²ā¶ØŹŌ¹ÜÖŠČÜŅŗµÄpH | ¢Ł | SO2+H2O H2SO3 H2SO3 |
| Óė¼ī ·“Ó¦ | ¢Ś | ³öĻÖ°× É«³Įµķ | ¢Ū | ||
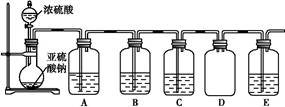
| ×°ÖĆ | Ņ©Ę· | ×÷ÓĆ |
| A | | ŃéÖ¤¶žŃõ»ÆĮņµÄ»¹ŌŠŌ |
| B | | |
| C | Ę·ŗģČÜŅŗ | |
(1)¢ŁŹŌ¹ÜÖŠŅŗĆęÉĻÉż,ČÜŅŗpH£¼7
¢Ś½«¶žŃõ»ÆĮņĘųĢåĶØČė×ćĮæ³ĪĒåŹÆ»ŅĖ®[»ņBa(OH)2ČÜŅŗ]ÖŠ
¢ŪSO2+Ca2++2OH-=CaSO3”ż+H2O(»ņSO2+Ba2++2OH-=BaSO3”ż+H2O)
(2)¢Ł×°ÖĆ Ņ©Ę· ×÷ÓĆ A ĖįŠŌøßĆĢĖį¼ŲČÜŅŗ B Na2SČÜŅŗ ŃéÖ¤¶žŃõ»ÆĮņµÄŃõ»ÆŠŌ C ŃéÖ¤¶žŃõ»ÆĮņµÄĘư׊Ō
¢Ś5SO2+2MnO4-+2H2O=2Mn2++5SO42-+4H+
¢ŪĘ·ŗģČÜŅŗĶŹÉ«
¢Ü·Ąµ¹Īü ²»ŗĻĄķ,ŅņĪŖ·¢ÉśµÄ·“Ó¦ĪŖ3Ba2++2NO3-+3SO2+2H2O=3BaSO4”ż+2NO”ü+4H+,Éś³ÉµÄNOČŌČ»¶Ō»·¾³ÓŠĪŪČ¾
½āĪö


 Ó„ÅɽĢøØĻĪ½Ó½Ģ²ÄŗÓ±±½ĢÓż³ö°ęÉēĻµĮŠ“š°ø
Ó„ÅɽĢøØĻĪ½Ó½Ģ²ÄŗÓ±±½ĢÓż³ö°ęÉēĻµĮŠ“š°ø ³õÖŠŹīĘŚĻĪ½ÓĻµĮŠ“š°ø
³õÖŠŹīĘŚĻĪ½ÓĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
ijŹµŃ銔×éĶ¬Ń§ĪŖĮĖĢ½¾æĶÓėÅØĮņĖįµÄ·“Ó¦£¬½ųŠŠĮĖČēĻĀŹµŃ飬ŹµŃé×°ÖĆČēĶ¼ĖłŹ¾”£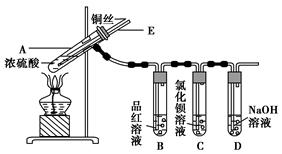
ŹµŃé²½Öč£ŗ
¢ŁĻČĮ¬½ÓČēĶ¼ĖłŹ¾µÄ×°ÖĆ£¬¼ģ²éŗĆĘųĆÜŠŌ£¬ŌŁ¼ÓČėŹŌ¼Į£»
¢Ś¼ÓČČAŹŌ¹Ü£¬“żBŹŌ¹ÜÖŠĘ·ŗģČÜŅŗĶĖÉ«ŗó£¬ĻØĆš¾Ę¾«µĘ£»
¢Ū½«CuĖæĻņÉĻ³é¶ÆĄėæŖŅŗĆę”£
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)AŹŌ¹ÜÖŠ·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ ”£
(2)Äܹ»Ö¤Ć÷ĶÓėÅØĮņĖį·“Ӧɜ³ÉĘųĢåµÄŹµŃéĻÖĻóŹĒ ”£
(3)ŌŚŹ¢ÓŠBaCl2ČÜŅŗµÄCŹŌ¹ÜÖŠ£¬³żĮĖµ¼¹ÜæŚÓŠĘųÅŻĶā£¬ĪŽĘäĖūĆ÷ĻŌĻÖĻó£¬Čō½«ĘäÖŠµÄČÜŅŗ·Ö³ÉĮ½·Ż£¬·Ö±šµĪ¼ÓĻĀĮŠČÜŅŗ£¬½«²śÉś³ĮµķµÄ»ÆѧŹ½ĢīČė±ķÖŠ¶ŌÓ¦µÄĪ»ÖĆ”£
| µĪ¼ÓµÄČÜŅŗ | ĀČĖ® | °±Ė® |
| ³ĮµķµÄ»ÆѧŹ½ | | |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
ij»Æѧъ¾æŠŌѧĻ°Š”×é¶ŌĮņĖįĶ·Ö½āĘųĢå²śĪļµÄ³É·Ö½ųŠŠČēĻĀĢ½¾æ:
”¾²éŌÄ׏ĮĻ”æ
ĮņĖįĶŹÜČČ·Ö½āÉś³ÉŃõ»ÆĶŗĶĘųĢå,ĪĀ¶Č²»Ķ¬Ź±,ĘųĢåæÉÄÜĪŖSO3,SO2ŗĶO2ÖŠµÄŅ»ÖÖ”¢Į½ÖÖ»ņČżÖÖ”£
”¾Ģį³ö¼ŁÉč”æ
¼ŁÉč1:ĘųĢå²śĪļÖ»ÓŠŅ»ÖÖ;
¼ŁÉč2:ĘųĢå²śĪļÖ»ÓŠĮ½ÖÖ;
¼ŁÉč3:ĘųĢå²śĪļÓŠČżÖÖ”£
(1)Čō¼ŁÉč1³ÉĮ¢,Ōņ²śĪļĘųĢåµÄ³É·ÖŹĒ””””””””;Čō¼ŁÉč2³ÉĮ¢,Ōņ²śĪļĘųĢåµÄ³É·ÖŹĒ”””””””””£
”¾ŹµŃé¼°½į¹ūĢÖĀŪ”æ
(2)¼×Ķ¬Ń§½«·Ö½āÉś³ÉµÄĘųĢåŅĄ“ĪĶعżŹ¢ÅØĮņĖįŗĶKMnO4ĖįŠŌČÜŅŗµÄĻ“ĘųĘæ,ÄÜĖµĆ÷²śĪļĘųĢåÖŠŗ¬SO2µÄĻÖĻóŹĒ ””,øĆĻÖĻóĖµĆ÷SO2¾ßÓŠµÄŠŌÖŹŹĒ””””””””,ŹµŃéÖŠ»¹·¢ĻÖŹ¢ÅØĮņĖįµÄĻ“ĘųĘæÖŹĮæĆ÷ĻŌŌö¼Ó,ŌŅņŹĒ”””””””””””””” ”””£
(3)ŅŅĶ¬Ń§½«·Ö½āÉś³ÉµÄĘųĢåĶعż¼īŹÆ»Ņŗó,ŌŁŹÕ¼Æ²ā¶ØŹ£ÓąĘųĢåµÄĢå»ż,ŌŚ²»Ķ¬ĪĀ¶ČĻĀ½ųŠŠ3×鏵Ń锣ĒėĶź³ÉĻĀ±ķ(ŹµŃéÖŠĮņĖįĶ¾łĶźČ«·Ö½ā):
| ŹµŃé ŠņŗÅ | ³ĘČ”CuSO4 µÄÖŹĮæ/g | ¼īŹÆ»ŅµÄ Ōö¼ÓÖŹĮæ/g | Ź£ÓąĘųĢåµÄĢå»ż(ÕŪĖć ³É±ź×¼×“æöĻĀ)/mL | ½įĀŪ |
| ¢ń | 6.4 | | | ¼ŁÉč1³ÉĮ¢ |
| ¢ņ | 6.4 | 2.88 | 224 | |
| ¢ó | 6.4 | 2.56 | 448 | |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
ŅŃÖŖAgNO3¾§Ģå¼ÓČČ·Ö½āÉś³ÉĮ½ÖÖµ„ÖŹŗĶŗģ×ŲÉ«ĘųĢ唣ÓĆĻĀĮŠÄ³Š©×°ÖĆ“ÖĀŌ²ā¶Ø»ģČėĮĖĘäĖū²»·Ö½āŅ²²»²ĪÓė·“Ó¦µÄŌÓÖŹµÄĻõĖįŅųµÄ“æ¶Č£¬²¢½ųŠŠÓŠ¹ŲŹµŃé(×°ÖĆÖŠ±ŲŅŖµÄĢś¼ÜĢØ”¢Ģś¼Š”¢¾Ę¾«µĘµČŅŃĀŌČ„)£¬ĢīŠ“ĻĀĮŠæÕ°×”£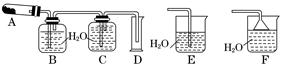
£Ø1£©Š“³öAgNO3ŹÜČČ·Ö½āµÄ»Æѧ·½³ĢŹ½£ŗ_______________________________________________________________”£
£Ø2£©²ā¶ØAgNO3µÄ“æ¶Č£¬æÉŃ”ÓĆÓÉA”¢B”¢C”¢D×é³ÉµÄ×°ÖĆ£¬µ«ĘäÖŠ²»ŗĻĄķµÄŹĒ____________£¬øĆ“ķĪóŅżĘšµÄŗó¹ūŹĒ____________________________________________”£
£Ø3£©BĘæÖŠµÄæÕĘų¶ŌŹµŃé½į¹ū________(Ģī”°ÓŠ”±»ņ”°ĪŽ”±)Ó°Ļģ£¬ĄķÓÉŹĒ____________________________________________________________________________”£
£Ø4£©Čē¹ūøĽų×°ÖĆŗ󣬳ĘČ”ĻõĖįŅųŹŌŃł4.00 gÖĆÓŚAÖŠ»ŗ»ŗ¼ÓČČ£¬“ż·“Ó¦ĶźČ«ŗ󣬲śÉśµÄĘųĢåĶعżB”¢C×°ÖĆŗ󣬲āµĆĮæĶ²ÖŠĖ®µÄĢå»ż£¬²¢ÕŪĖć³É±ź×¼×“æöĻĀĘųĢåµÄĢå»żĪŖ112 mL£¬ŌņŹŌŃłµÄ“æ¶ČĪŖ________”£
£Ø5£©Čē¹ū¼ÓČČCu(NO3)2Éś³ÉŃõ»ÆĶ£¬Ōņ¼ÓČČ·Ö½āĖłµĆĘųĢåÓ¦ĶØČė×°ÖĆ________(Ģī”°E”±»ņ”°F”±)£¬ĘäĄķÓÉŹĒ__________________________________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
Įņ“śĮņĖįÄĘ£ØNa2S2O3£©æÉÓÉŃĒĮņĖįÄĘŗĶĮņ·ŪĶعż»ÆŗĻ·“Ó¦ÖʵƔ£ŅŃÖŖ£ŗNa2S2O3ŌŚĖįŠŌČÜŅŗÖŠ²»ÄÜĪČ¶Ø“ęŌŚ”£
£Ø1£©Ä³ŃŠ¾æŠ”×éÉč¼ĘµÄÖʱøNa2S2O3”¤5H2OµÄ×°ÖĆŗĶ²æ·Ö²Ł×÷²½ÖčČēĻĀ”£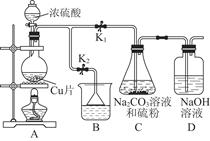
¢ń.“ņæŖK1¹Ų±ÕK2£¬ĻņŌ²µ×ÉÕĘæÖŠ¼ÓČė×ćĮæÅØĮņĖį£¬¼ÓČČ”£
¢ņ.CÖŠ»ģŗĻŅŗ±»ĘųĮ÷½Į¶Æ£¬·“Ó¦Ņ»¶ĪŹ±¼äŗó£¬Įņ·ŪµÄĮæÖš½„¼õÉŁ£¬µ±CÖŠČÜŅŗµÄpH ½Ó½ü7Ź±£¬“ņæŖK2£¬¹Ų±ÕK1¼“Ķ£Ö¹CÖŠµÄ·“Ó¦£¬Ķ£Ö¹¼ÓČČ”£
¢ó.¹żĀĖCÖŠµÄ»ģŗĻŅŗ”£
¢ō.½«ĀĖŅŗ¾¹ż ”¢ ”¢¹żĀĖ”¢Ļ“µÓ”¢ŗęøÉ£¬µĆµ½²śĘ·Na2S2O3”¤5H2O”£
¢Ł¢ņÖŠ£¬”°µ±CÖŠČÜŅŗµÄpH½Ó½ü7Ź±¼“Ķ£Ö¹CÖŠµÄ·“Ó¦”±µÄŌŅņŹĒ £ØÓĆĄė×Ó·½³ĢŹ½±ķŹ¾£©”£
¢Ś¢ōÖŠ£¬²Ł×÷²½ÖčŹĒ ”¢ ”£
¢Ū×°ÖĆBÖŠŹ¢·ÅµÄŹŌ¼ĮŹĒ£ØĢī»ÆѧŹ½£© ČÜŅŗ”£
¢ÜÓŠŅ»Š”×éŌŚŹµŃéÖŠ·¢ĻÖ£¬¶žŃõ»ÆĮņĘųĢå²śÉś»ŗĀżŅŌÖĀŗóŠųĻÖĻó²»Ć÷ĻŌ£¬µ«ÓÖ²»“ęŌŚĘųĆÜŠŌĪŹĢā£¬ĒėÄćĶĘ²āæÉÄܵÄŌŅņ ”£
£Ø2£©³£ÓĆNa2S2O3ČÜŅŗ²ā¶Ø·ĻĖ®ÖŠBa2£«µÄÅØ¶Č£¬²½ÖčČēĻĀ£ŗČ”·ĻĖ®25.00 mL£¬æŲÖĘŹŹµ±µÄĖį¶Č¼ÓČė×ćĮæ K2Cr2O7ČÜŅŗ£¬µĆBaCrO4³Įµķ£»¹żĀĖ”¢Ļ“µÓŗó£¬ÓĆŹŹĮæĻ”ŃĪĖįČܽā£¬“ĖŹ±CrO42-Č«²æ×Ŗ»ÆĪŖCr2O72-£»ŌŁ¼Ó¹żKIČÜŅŗ£¬³ä·Ö·“Ó¦ŗóµĆ»ģŗĻČÜŅŗV mL£¬½«ĘäĘ½¾ł·Ö³É4µČ·Ż£¬¼ÓČėµķ·ŪČÜŅŗ×÷ÖøŹ¾¼Į£¬ÓĆ0.001 0 mol”¤L£1µÄNa2S2O3ČÜŅŗ½ųŠŠµĪ¶Ø£¬·“Ó¦ĶźČ«Ź±£¬Ļą¹ŲŹż¾Ż¼ĒĀ¼ČēĻĀ±ķĖłŹ¾£ŗ
| ±ąŗÅ | 1 | 2 | 3 | 4 |
| ĻūŗÄNa2S2O3±ź×¼ | | | | |
| ČÜŅŗµÄĢå»ż/mL | 18.02 | 17.98 | 18.00 | 20.03 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
¹¤ŅµÉĻ³£ÓĆĢśÖŹČŻĘ÷Ź¢×°ĄäÅØĮņĖį”£ĪŖŃŠ¾æĢśÖŹ²ÄĮĻÓėČČÅØĮņĖįµÄ·“Ó¦£¬Ä³Ń§Ļ°Š”×é½ųŠŠĮĖŅŌĻĀĢ½¾æ»ī¶Æ£ŗ
”¾Ģ½¾æŅ»”æ
£Ø1£©½«ŅŃČ„³ż±ķĆęŃõ»ÆĪļµÄĢś¶¤£ØĢ¼ĖŲøÖ£©·ÅČėĄäÅØĮņĖįÖŠ£¬10·ÖÖÓŗóŅĘČėĮņĖįĶČÜŅŗÖŠ£¬Ę¬æĢŗóČ”³ö¹Ū²ģ£¬Ģś¶¤±ķĆęĪŽĆ÷ĻŌ±ä»Æ£¬ĘäŌŅņŹĒ__________________”£
£Ø2£©ĮķČ”Ģś¶¤6.0 g·ÅČė15.0 mLÅØĮņĖįÖŠ£¬¼ÓČČ£¬³ä·Ö·“Ó¦ŗóŹÕ¼Æµ½ĘųĢåY”£
¼×Ķ¬Ń§Č”336 mL£Ø±ź×¼×“æö£©ĘųĢåYĶØČė×ćĮæäåĖ®ÖŠ£¬·¢Éś·“Ó¦£ŗSO2+Br2+2H2O=2HBr+H2SO4
Č»ŗó¼ÓČė×ćĮæBaCl2ČÜŅŗ£¬¾ŹŹµ±²Ł×÷ŗóµĆøÉŌļ¹ĢĢå2.33 g”£ÓÉ“ĖĶĘÖŖĘųĢåYÖŠSO2µÄĢå»ż·ÖŹżĪŖ______”£
”¾Ģ½¾æ¶ž”æ
·ÖĪöÉĻŹöŹµŃéÖŠSO2Ģå»ż·ÖŹżµÄ½į¹ū£¬ŅŅĶ¬Ń§ČĻĪŖĘųĢåYÖŠ»¹æÉÄÜŗ¬ÓŠH2ŗĶQĘųĢ唣ĪŖ“ĖÉč¼ĘĮĖĻĀĮŠĢ½¾æŹµŃé×°ÖĆ£ØĶ¼ÖŠ¼Š³ÖŅĒĘ÷Ź”ĀŌ£©”£
£Ø3£©×°ÖĆBÖŠŹŌ¼ĮµÄ×÷ÓĆŹĒ_________________________________________”£
£Ø4£©ČĻĪŖĘųĢåYÖŠ»¹ŗ¬ÓŠQµÄĄķÓÉŹĒ______________________________”££ØÓĆ»Æѧ·½³ĢŹ½±ķŹ¾£©”£
£Ø5£©ĪŖČ·ČĻQµÄ“ęŌŚ£¬ŠčŌŚ×°ÖĆÖŠĢķ¼ÓMÓŚ______£ØŃ”ĢīŠņŗÅ£©”£
a.AÖ®Ē° b.A”¢B¼ä c.B”¢C¼ä d.C”¢D¼ä
£Ø6£©Čē¹ūĘųĢåYÖŠŗ¬ÓŠH2£¬Ō¤¼ĘŹµŃéĻÖĻóÓ¦ŹĒ____________________________”£
£Ø7£©ČōŅŖ²ā¶ØĻŽ¶ØĢå»żĘųĢåYÖŠH2µÄŗ¬Įæ£Ø±ź×¼×“æöĻĀŌ¼ÓŠ28 mL H2£©£¬³żæÉÓĆ²āĮæH2Ģå»żµÄ·½·ØĶā£¬æÉ·ńŃ”ÓĆÖŹĮæ³ĘĮæµÄ·½·Ø£æ×ö³öÅŠ¶Ļ²¢ĖµĆ÷ĄķÓÉ________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
ij»ÆѧŠĖȤŠ”×éµÄĶ¬Ń§ĄūÓĆĻĀĶ¼ĖłŹ¾ŹµŃé×°ÖĆ½ųŠŠÄ³Š©ĘųĢåµÄÖʱø”¢ŠŌÖŹµČŹµŃé(Ķ¼ÖŠ¼Š³Ö×°ÖĆÓŠŹ”ĀŌ)”£Ēė°“ŅŖĒóĢīæÕ£ŗ
¢ń.Ģ½¾æĀČĘųÓė°±ĘųµÄ·“Ó¦
(1)ĪŖÖĘČ”øÉŌļ°±Ęų£¬æɽ«×°ÖĆCÓė________(Ģī×°ÖƱąŗÅ)Į¬½Ó£»×°ÖĆCÖŠµÄÉÕĘæÄŚ¹ĢĢåŅĖŃ”ÓĆ________”£
a£®¼īŹÆ»Ņ b£®ĀČ»ÆøĘ c£®ĪåŃõ»Æ¶žĮ× d£®ÉśŹÆ»Ņ
(2)×°ÖĆA”¢E”¢EĮ¬½ÓæÉÖĘČ”“æ¾»”¢øÉŌļµÄĀČĘų£¬ŌņĮ½øöE×°ÖĆÄŚµÄŅ©Ę·ŅĄ“ĪŹĒ________________”£
(3)×°ÖĆFæÉÓĆÓŚĢ½¾æĀČĘųÓė°±Ęų(ŅŃÖŖĀČĘųÓė°±ĘųæÉ·¢Éś·“Ó¦£ŗ3Cl2£«2NH3===N2£«6HCl)µÄ·“Ó¦”£ŹµŃ鏱“ņæŖµÆ»É¼Š1”¢3£¬¹Ų±Õ2£¬ĻČĻņÉÕĘæÖŠĶØČė________£¬Č»ŗó¹Ų±Õ1”¢3£¬“ņæŖ2£¬ĻņÉÕĘæÖŠ»ŗĀżĶØČėŅ»¶ØĮæµÄĮķŅ»ÖÖĘųĢ唣ŹµŃéŅ»¶ĪŹ±¼äŗóÉÕĘæÄŚ³öĻÖÅØŗńµÄ°×ŃĢ²¢ŌŚČŻĘ÷ÄŚ±ŚÄż½į£¬ĒėÉč¼ĘŅ»øöŹµŃé·½°ø¼ų¶ØøĆ¹ĢĢåÖŠµÄŃōĄė×Ó______________________________________________________________________________”£
¢ņ.Ģ½¾æijŠ©ĪļÖŹµÄŠŌÖŹ
(4)ĄūÓĆ×°ÖĆA”¢E£¬æÉÉč¼ĘŹµŃé±Č½ĻCl£ŗĶBr£µÄ»¹ŌŠŌĒæČõ£¬ÄÜÖ¤Ć÷½įĀŪµÄŹµŃéĻÖĻóŹĒ______________________________________________________”£
(5)ČōĄūÓĆ×°ÖĆA”¢E½ųŠŠŅŅĻ©ÓėäåĖ®·“Ó¦µÄŹµŃ飬Š“³ö·“Ó¦µÄ»Æѧ·½³ĢŹ½________________________________________________________________”£
(6)½«×°ÖĆB”¢C·Ö±šÓėFĻąĮ¬ŗ󣬽ųŠŠH2SÓėSO2·“Ó¦µÄŹµŃ锣FµÄÉÕĘæÖŠ·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ______________________£»FµÄÉÕ±ĖłĘšµÄ×÷ÓĆŹĒ________________________________________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
Ēė·ÖĪö»Ų“šÄ³Ķ¬Ń§ŌŚĢ½¾æÅØĮņĖį”¢Ļ”ĮņĖį”¢ÅØĻõĖį”¢Ļ”ĻõĖį·Ö±šÓėĶ·“Ó¦µÄŹµŃéÖŠ·¢ĻÖµÄÓŠ¹ŲĪŹĢā”£
¢ń.Ģ½¾æÉĻŹöĖÄÖÖĖįµÄŃõ»ÆŠŌĻą¶ŌĒæČõ¼°ĘäÓėĶ·“Ó¦µÄ»¹Ō²śĪļµÄŠŌÖŹ
(1)·Ö±šĻņŹ¢ÓŠµČĮæĶʬµÄĖÄÖ§ŹŌ¹ÜÖŠ¼ÓČėµČĢå»żµÄÅØĮņĖį”¢Ļ”ĮņĖį”¢ÅØĻõĖį”¢Ļ”ĻõĖį,ŹµŃé½į¹ū¼ĒĀ¼ČēĻĀ±ķ:
| | Ėį | ŹµŃé½į¹ū |
| a | ÅØĮņĖį | ¼ÓČČŗó·¢Éś·“Ó¦,²śÉśĪŽÉ«“Ģ¼¤ŠŌĘųĢå |
| b | Ļ”ĮņĖį | ¼ÓČČŅ²²»·¢Éś·“Ó¦ |
| c | ÅØĻõĖį | ²»¼ÓČČ¼“·¢Éś·“Ó¦,²śÉśŗģ×ŲÉ«ĘųĢå |
| d | Ļ”ĻõĖį | Ī¢ČČ·¢Éś·“Ó¦,²śÉśĪŽÉ«ĘųĢå |


²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
ijĶ¬Ń§ÓĆĻĀĆęµÄ×°ÖĆÖʱø²¢ŹÕ¼Æ“æ¾»µÄĀČ»ÆĢś£¬Ó²ÖŹ²£Į§¹ÜE֊װӊĻøĢśĖæĶų”£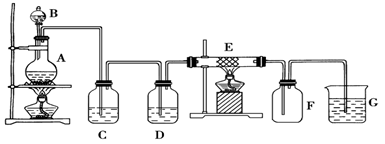
ŹŌ»Ų“š£ŗ
£Ø1£©¼ģŃé×°ÖĆAµÄĘųĆÜŠŌµÄ·½·ØŹĒ
£Ø2£©×°ÖĆAÖŠ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ
£Ø3£©×°ÖĆCµÄ×÷ÓĆŹĒ£ŗ ________________£¬×°ÖĆDÖŠµÄŹŌ¼ĮŹĒ£ŗ____ _______”£
£Ø4£©æɼģŃé×°ÖĆEÖŠÉś³ÉµÄĪļÖŹÖŠŃōĄė×ӵķ½·Ø¼°ĻÖĻóŹĒ ”£
£Ø5£©Čō°ŃĀČĘųĶØČėŹÆČļČÜŅŗÖŠ£¬¹Ū²ģµÄĻÖĻóŹĒ£ŗ ”£
£Ø6£©×°ÖĆ GÖŠ·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ£ŗ________________ _ ____________”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com