
�ж�������ȷ�Ļ����̡�������Ļ�������
(1)S(s)�� O2(g)===SO3(g)����H����315 kJ·mol��1(ȼ����)��(��H����ֵ��ȷ)(����)
O2(g)===SO3(g)����H����315 kJ·mol��1(ȼ����)��(��H����ֵ��ȷ)(����)
(2)NaOH(aq)��HCl(aq)===NaCl(aq)��H2O(l)
��H����57.3 kJ·mol��1(�к���)��(��H����ֵ��ȷ)(����)
(3)��֪H��(aq)��OH��(aq)===H2O(l)����H����57.3 kJ·mol��1����H2SO4��Ba(OH)2��Ӧ�ķ�Ӧ�Ȧ�H��2��(��57.3) kJ·mol��1(����)
(4)ȼ�ϵ���н��״�����ת��Ϊ�������Ȼ�ѧ����ʽ��CH3OH(g)�� O2(g)===CO2(g)��2H2(g)
O2(g)===CO2(g)��2H2(g)
��H����192.9 kJ·mol��1����CH3OH(g)��ȼ����Ϊ192.9 kJ·mol��1(����)
(5)H2(g)��ȼ������285.8 kJ·mol��1����2H2O(g)===2H2(g)��O2(g)����H����571.6 kJ·mol��1(����)
(6)�����ǵ�ȼ������2 800 kJ·mol��1���� C6H12O6(s)��3O2(g)===3CO2(g)��3H2O(l)����H����1 400 kJ·mol��1(����)
C6H12O6(s)��3O2(g)===3CO2(g)��3H2O(l)����H����1 400 kJ·mol��1(����)
(7)��֪101 kPaʱ��2C(s)��O2(g)===2CO(g)
��H����221 kJ·mol��1����÷�Ӧ�ķ�Ӧ��Ϊ221 kJ·mol��1(����)
(8)��֪ϡ��Һ�У�H��(aq)��OH��(aq)===H2O(l)
��H����57.3 kJ·mol��1����ϡ������ϡ����������Һ��Ӧ����1 molˮʱ�ų�57.3 kJ������(����)
(9)��֪HCl��NaOH��Ӧ���к��Ȧ�H����57.3 kJ·mol��1����98%��Ũ������ϡ����������Һ��Ӧ����1 molˮ���к���Ϊ��57.3 kJ·mol��1(����)
(10)CO(g)��ȼ������283.0 kJ·mol��1����2CO2(g)===2CO(g)��O2(g)��Ӧ�Ħ�H����2��283.0 kJ·mol��1(����)
(11)������ȼ����Ϊ285.5 kJ·mol��1������ˮ���Ȼ�ѧ����ʽΪ2H2O(l)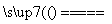 2H2(g)��O2(g)����H����285.5 kJ·mol��1(����)
2H2(g)��O2(g)����H����285.5 kJ·mol��1(����)
 Ӣ��СӢ������Ĭдϵ�д�
Ӣ��СӢ������Ĭдϵ�д� �����ҵ���������ͯ������ϵ�д�
�����ҵ���������ͯ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��0 �桢1.01��105 Paʱ��������ʵ�飺�ס��ҡ�����λͬѧ��ȡ30.0 mLͬŨ�ȵ����ᣬ������ͬ��ɵ�þ���Ͻ��ĩ���ⶨ���������������й������б����£�
| ʵ����� | �� | �� | �� |
| �Ͻ�����(mg) | 510 | 765 | 918 |
| �������(mL) | 560 | 672 | 672 |
����(1)��������ʵ���Ũ���Ƕ��٣�
(2)�Ͻ��и��ɷֵ�����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ������í���̶�����ͭ�������ʾ��ͼ������������ڳ�ʪ�����У�����˵����ȷ����(����)
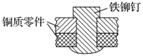
A�������绯ѧ��ʴ��ͭΪ������ͭ������H2
B��ͭ�ױ���ʴ��ͭ���Ϸ�����ԭ��Ӧ������O2
C�����ױ���ʴ��������������Ӧ��Fe��2e===Fe2��
D��������ѧ��ʴ��Fe��Cu2��===Cu��Fe2��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ͨ���Ѳ�1 molij��ѧ�������յ��������ɸû�ѧ���ļ��ܡ����ܵĴ�С���Ժ�����ѧ����ǿ����Ҳ�����ڹ��㻯ѧ��Ӧ�ķ�Ӧ��(��H)����ѧ��Ӧ�Ħ�H���ڷ�Ӧ�ж��Ѿɻ�ѧ���ļ���֮���뷴Ӧ���γ��»�ѧ���ļ���֮�͵IJ�����о���һЩ��ѧ���ļ������ݣ�������ʹ�á�
| ��ѧ�� | Si—O | Si—Cl | H—H | H—Cl | Si—Si | Si—C |
| ����/kJ·mol��1 | 460 | 360 | 436 | 431 | 176 | 347 |
��ҵ�ϵĸߴ����ͨ�����з�Ӧ��ȡ��SiCl4(g)��2H2(g)===Si(s)��4HCl(g)���÷�Ӧ�ķ�Ӧ�Ȧ�HΪ______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ж������Ȼ�ѧ����ʽ��д�Ƿ���ȷ����ȷ�Ļ����̡�������Ļ�������(ע���ʱ����ݾ���ȷ)
(1)CaCO3(s)===CaO��CO2(g)����H����177.7 kJ(����)
(2)C(s)��H2O(s)===CO(g)��H2(g)����H����131.3 kJ·mol��1(����)
(3)C(s)�� O2(g)===CO(g)
O2(g)===CO(g)
��H����110.5 kJ·mol��1(����)
(4)CO(g)�� O2(g)===CO2(g)
O2(g)===CO2(g)
��H����283 kJ·mol��1(����)
(5)2H2(g)��O2(g)===2H2O(l)
��H����571.6 kJ·mol��1(����)
(6)500 �桢30 MPa�£���0.5 mol N2(g)��1.5 mol H2(g)�����ܱ������г�ַ�Ӧ����NH3(g)������
19��3 kJ�����Ȼ�ѧ����ʽΪN2(g)��3H2(g) 2NH3(g)����H����38.6 kJ·mol��1(����)
2NH3(g)����H����38.6 kJ·mol��1(����)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������ϵ�IJ�ͬ��Ӧ���
C(s)��O2(g)===CO2(g)����H1<0
C(s)�� O2(g)===CO(g)����H2<0
O2(g)===CO(g)����H2<0
��H1____��H2��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��Դ�������ִ���ᷢչ�������������֮һ����Դ�����У���ú̿���ں��ܡ���ʯ�͡�����Ȼ������ˮ�ܡ����ܡ��ߵ����ܵȣ����ڲ�����������________(����ţ���ͬ)����������Դ����________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪��C(s)��O2(g)===CO2(g)����H1
CO2(g)��C(s)===2CO(g)����H2
2CO(g)��O2(g)===2CO2(g)����H3
2Cu(s)��O2(g)===2CuO(s)����H4
CO(g)��CuO(s)===CO2(g)��Cu(s)����H5
���й���������Ӧ�ʱ���ж���ȷ����(����)
A����H1>0����H3<0 B����H2<0����H4>0
C����H2����H1����H3 D����H3����H4����H5
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵����ȷ����(����)
A�������£�ij��Һ����ˮ�������c(H��)��1��10��amol·L��1����a>7ʱ�������Һ��pHһ��Ϊ14��a
B��pH��9��CH3COONa��Һ��pH��9��NH3·H2O��Һ������Һ��ˮ�ĵ���̶���ͬ
C���¶�һ��ʱ����ˮ�еμ����������γ�ϡ��Һ��ˮ�����ӻ�����Kw����
D�������£���pH��4�Ĵ�����Һϡ�ͺ���Һ���������ӵ�Ũ�Ⱦ�����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com