


 ���Ž�������С״Ԫϵ�д�
���Ž�������С״Ԫϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

��һ��С�ձ������Լ
������������⣺
��1��д����Ӧ�Ļ�ѧ����ʽ��____________________________________________��
��2��ʵ����Ҫ�����ò�����Ѹ�ٽ����ԭ����______________________________________��
��3�����ʵ����û����������������ܵ�ԭ����__________________________________
____________________________________________________________________��(�����������������ԭ��)��
��4�����û�п�����������������ǻ����Բ�ȡ��Щ��ʽ��˵���÷�Ӧ���ȣ�
______________________________________________________________��������ַ�������
��5��ʵ���м�ʹ����������������ձ���ʱ�����ձ��벣��ƬҲ��ճ��һ���ˡ���ԭ����____________________________________________________________________��
��6�����üķ���˵���ձ��벣��Ƭ֮������Ϊ���������ճ��һ��ģ�_________________
__________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�����ʡ����һ�и�һ��ѧ�ڵڶ��νβ��Ի�ѧ�Ծ����������� ���ͣ������
��Դ�������������ᷢչ�Ļ������о���ѧ��Ӧ�е������仯�������ڸ��õ����û�ѧ��ӦΪ��������������Ķ������й���Դ�IJ��ϣ��ش��й����⣺
��1���������ĽǶȿ����Ͽ���ѧ��Ҫ�����������γɻ�ѧ��Ҫ�ų���������֪��1 mol H��H����1 mol I��I��1 mol H��I���ֱ���Ҫ���յ�����Ϊ436 kJ��151 kJ��299 kJ�����������͵ⵥ�ʷ�Ӧ��H2+I2=2HI������1 mol HI��Ҫ ����ų��������ա��� ___ kJ��������
��2���������������о���������ѧ������ܵ��ת����
����ͼ��װ���У������缫��ӦʽΪ ���������� ��Ӧ�����������ԭ������ͬ���������缫��ӦʽΪ ���ܷ�Ӧ�����ӷ���ʽΪ ��
��3����������������Һ���������Һ����������ȼ�ϣ��������������Ƴ�ȼ�ϵ�أ��为����ӦʽΪ ��������ӦʽΪ �����б�״��2.24L��������ʱ����һ���������������ʵ���Ϊ ��
��4��������ͼ��ʾ��ԭ����У����� ���������ϵ����� ������ ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012�꽭����ͨС����ѧ��һ��ѧ�����п��Ի�ѧ�Ծ����������� ���ͣ������
��13�֣���Դ�������������ᷢչ�Ļ������о���ѧ��Ӧ�е������仯�������ڸ��õ����û�ѧ��ӦΪ��������������Ķ������й���Դ�IJ��ϣ��ش��й����⣺
��1���������ĽǶȿ����Ͽ���ѧ��Ҫ ���γɻ�ѧ��Ҫ ��
��2���������������о���������ѧ������ܵ��ת����
��ͼ���ǽ� ��ת��Ϊ �ܵ�װ�ã������缫��ӦʽΪ ������______��Ӧ�����������ԭ������ͬ������������ ������______��Ӧ;������Ӧʱ��������_____Ƭ����_____Ƭ������������2 mol����ͨ��ʱ������������ g ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015��ɽ��ʡΫ���и߶���ѧ����ĩͳ����ѧ�Ծ��������棩 ���ͣ������
�о���ѧ��Ӧ�е������仯����Ҫ���塣�����ѧ��֪ʶ�ش��������⣺
��1����֪һ����̼��ˮ������Ӧ���̵������仯����ͼ��ʾ��
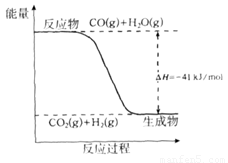
�ٷ�Ӧ���Ȼ�ѧ����ʽΪ____________________________________________��
����֪��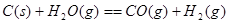
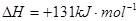
��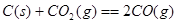
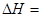
��2����ѧ��Ӧ����Ϊ�ɼ����Ѻ��¼��γɵĹ��̡���ѧ���ļ������γɣ����1 mol��ѧ��ʱ�ͷţ������գ�����������֪��N��N���ļ�����948.9kJ��mol��1��H��H���ļ�����436.0 kJ��mol��1�� N��H���ļ�����391.55 kJ��mol��1����1/2N2(g) + 3/2H2(g) == NH3(g) ��H = ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com