
��14�֣�ʵ����
��ͼ��ijѧУʵ���Ҵӻ�ѧ�Լ��̵���ص�Ũ�����Լ���ǩ�ϵIJ������ݡ����ø�Ũ��������200 mL 1.0 mol/L��ϡ���ᡣ�ɹ�ѡ�õ������У�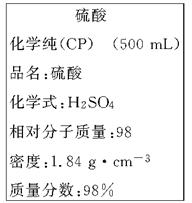
�ٲ�����������ƿ�����ձ�����ҩ�ף�����Ͳ����������ƽ��
��ش��������⣺
��1����������ϡ����ʱ����ȱ�ٵ�������________________________(д��������)��
��2����ǩ��ʾŨ��������ʵ���Ũ��Ϊ___________________________
��3������200 mL 1.0 mol/L��ϡ������Ҫ����Ͳ��ȡ����Ũ��������Ϊ_______mL������������С�����1λ������ȡ����ʱӦѡ��_______������Ͳ��
| A��10 mL | B��50 mL |
| C��100 mL | D��200 mL |
��1��250mL����ƿ����ͷ�ι�
��2��18.4mol/L ��3��13.6 B ��4���ڢۢܣ���5���٢�
������������� ��1�����������м��㡢��ȡ��ϡ�͡���Һ��ϴ����Һ�����ݡ�ҡ�ȵȲ���������Ͳ��ȡ���õ���ͷ�ιܣ�Ũ���ᣬ���ձ���ϡ�ͣ��ò��������裬��ȴ��ת�Ƶ�250mL����ƿ��ʵ������û��200mL����ƿ���У����ò�����������ϴ��2-3�Σ���ϴ��Һת�Ƶ�����ƿ�У���ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�����ݵߵ�ҡ�ȡ����ṩ��������֪����Ҫ�����У�250mL����ƿ����ͷ�ιܡ�
��2��Ũ��������ʵ���Ũ��Ϊ��1000��wM=1000��1.84��98%98mol/L=18.4mol/L��
��3������200mL 1.0mol/L��ϡ���ᣬʵ�������Ƶ���250mL 1.0mol/L��ϡ���ᣬ��ҪŨ��������Ϊ��(1.0mol/L��0.25L)��18.4mol/L��0.0136L="13.6" mL����Ҫѡ��50mL��Ͳ������B��ȷ��
��4��������Ͳ��ȡ�������������Ũ���ᣬ����ȷ������Ͳ��������ϡ����Һ���ڴ���Ũ����ϡ�ͺ���ȣ�Ӧ�ָ������º��ٽ���ϡ�ͺ����Һת������ƿ�У��۴�������ˮ��Һ�����̶���1��2cm�������ý�ͷ�ι�С�ĵμ�����ˮ����Һ��Һ����͵���̶������У��ܴ��ݰ�����ƿ�Ǹǽ�����ҡ�ȣ�����ȷ����ѡ���ڢۢܡ�
��5��������Ͳ��ȡŨ����ʱ�����ӿ̶���ȡŨ���ᣬ��ȡŨ������������������ҺŨ��ƫ�ߣ��ٿ�ѡ���������Ҫ���ݣ�����ƿ�����������������ˮ������ҺŨ����Ӱ�죬�ڲ�ѡ����ϴ���ձ��ڱں�ϴ��Һ��ȥ��������ʧ��Ũ��ƫ�ͣ��۲�ѡ����ת����Һʱ��������������Һ��������ƿ���棬��������ƿ�е�������������ʵ�����С��������ҺŨ��ƫ�ͣ��ܲ�ѡ���ݶ���ʱ����������ƿ�̶��ߣ�����������Һ�����С��������ҺŨ��ƫ�ߣ��ݿ�ѡ������ҡ�Ⱥ���Һ����ڿ̶��ߣ������ٴμ�������ˮ����Ӱ�����ƽ������ѡ����ѡ���٢ݡ�
���㣺����һ�����ʵ���Ũ����Һ�����ơ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��20�֣����к�������NaCl��Na2SO4��Na2CO3�����ʵ�NaNO3��Һ��ѡ���ʵ����Լ���ȥ���ʣ��õ�������NaNO3���壬ʵ����������ͼ��ʾ��
��1������A����Ҫ�ɷ��� ���ѧʽ����
��2�����з�Ӧ�����ӷ���ʽ�� ��
��3���٢ڢ��о����еķ�������� ��
��4����Һ3�����������Եõ�NaNO3���壬��Һ3�п϶����е������� ��Ϊ�˳�ȥ���ʣ�������Һ3�м��������� ��
��5��ʵ����������ʵ���õ�NaNO3��������500 mL 0.40 mol/L NaNO3��Һ��
��������Һʱ���������²�����a�����ݣ�b�����㣻c���ܽ⣻d��ҡ�ȣ�e��ת�ƣ�f��ϴ�ӣ�j����������ȡNaNO3����������� g��
���ղ���˳��4���� ������ţ���
��ijͬѧת����Һ�IJ�����ͼ��ʾ����ͬѧ�����еĴ����� ��
�����ý�ͷ�ιܶ���ʱ����С�ĵ�ˮ�ι��˿̶��ߣ�����ΪӦ�ò�ȡ�Ĵ��������ǣ� ��
�����в����У����������������Һ��Ũ��ƫ�͵��� ����ѡ���
a��û��ϴ���ձ��Ͳ�����
b������ʱ�����ӿ̶���
c��ϴ�Ӻ������ƿ�в�����������ˮ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��12�֣�ʵ�������ܶ�Ϊ1��18g/mL����������Ϊ36��5%Ũ��������250mLO��lmol/L��ϡ������Һ��ղ���ش��������⣺
��1��ʹ������ƿǰ������е�һ��������______________________________��
��2������250mL0��lmol/L����ϡ����Һʱ������ȷ�IJ���˳���ǣ���ĸ��ʾ��ÿ����ĸֻ����һ�Σ�________________________________________��
| A����30mLˮϴ���ձ�2��3�Σ�ϴ��Һ��ע������ƿ���� |
| B������Ͳȷ��ȡ����Ũ�������__________mL���ز����������ձ��У��ټ�������ˮ(Լ30mL)���ò���������������ʹ���Ͼ��� |
| C��������ȴ�������ز�����ע��mL������ƿ�� |
| D��������ƿ�ǽ�����ҡ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
����13�֣�ʵ������Ҫ90mL 2.0 mol��L��1��Na2CO3��Һ��������ˮ̼���Ʒ�δ���ƣ���ش��������⣺
��1������ͨ�����㣨Ҫ�м�����̣�����ȷ����ȡ g��ˮ̼���ơ�
��2�����������У������õ�����
A��50mL����ƿ�� B��100mL����ƿ�� C��������
D��100mL��Ͳ�� E��������ƽ�� F��ҩ��
��3����Ҫʵʩ���ƣ������������⣬��ȱ�������� ��
��4������ƿ��ʹ��ǰ������еIJ����� ��
��5�����ƹ��̼���Ϊ���¸���������ȷ�IJ���˳��Ϊ (����������)��
A����ȴ�����£� B��ϴ�Ӳ���Һ�� C����ȡ�� D���ܽ⣻
E��ҡ��װƿ�� F�����ݣ� G����Һ
��6�������ƹ����У����������Ũ���к�Ӱ��?
������ƿ������ˮϴ����û�ȵ������������Һ���ݣ���������Һ��Ũ�� ���ƫ�ߡ���ƫ�͡�����Ӱ�족����ͬ����
�� ת����Һʱ����С������Һ����ƿ�⣬��������Һ��Ũ��
�۶���ʱ�����ӿ̶��ߣ���������Һ��Ũ��
�����ڵμ�����ˮʱ�����������˿̶��ߣ���������Һ��Ũ�� ����ʱӦ��δ����� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��13�֣���ʾ��ҺŨ�ȵķ���ͨ��������:��Һ�����ʵ���������(��)�����ʵ���Ũ��(c)�������������Һʱ�����ݲ�ͬ����Ҫ���в�ͬ�����Ʒ������������������⡣
��1����10��(�ܶ�Ϊ1.00g��cm-3)��NaOH��Һ���Ƴ�27.5g2����NaOH��Һ��
�ټ��㣺��______g10��(�ܶ�Ϊ1.00g��cm-3)��NaOH��Һ�����______mLˮ(�ܶ�Ϊ1.00g��cm-3)����ϡ�͡�
����ȡ����_______mL��Ͳ���ɹ�ѡ�����Ͳ����У�5mL��10mL��25mL��50mL����ͬ����ȡ10����NaOH��Һ����ȡʱ����Ҫ����Ͳ��Һ��_______���У�Ȼ�����ձ����______mL��Ͳ��ȡ����ˮע���ձ��
��2����98��(�ܶ�Ϊ1.84g��cm-3)��Ũ����ϡ�ͳ�3mol/L��ϡ����100mL���ش��������⣺
����Ҫ��ȡŨ����___________mL(����һλС��)��
�����Ʋ����ɷֽ�����¼�������ȷ�IJ���˳���ǣ�___________��
A��������ƿ��ע����������ˮ������Ƿ�©ˮ
B������������ˮϴ���ձ�������������ϴ��Һע������ƿ�У����ظ���������
C��������ȴ��ϡ����ע�뾭��鲻©ˮ������ƿ��
D�����ݼ��㣬����Ͳ��ȡһ�������Ũ����
E����Ũ�������ձ�������ע��ʢ������ˮ��С�ձ��У��������ò���������
F����������ƿ�����ӣ���ҡ��
G���ý�ͷ�ιܵμ�����ˮ��ʹҺ��ﵽ�̶���
H������������ƿ�м�����ˮ��ʹҺ��ӽ��̶���
���������C�����а�δ��ȴ��ϡ����ע������ƿ�У�������Һ��Ũ�Ƚ�_________ (�ƫ�ߡ�����ƫ�͡�����Ӱ�족����ͬ)���������D��������ȡŨ�������Ͳ��������Ũ��������������ˮϴ�Ӳ���ϴ��Һת��E�����е�С�ձ��У�������Һ��Ũ�Ƚ�_________���������G������Ŀ�����ӣ�������Һ��Ũ�Ƚ�_________���������D������Ŀ�⸩�ӣ�������Һ��Ũ�Ƚ�_________��
��3��ʵ������NaOH��������1mol/L��NaOH��Һ����98��(�ܶ�Ϊ1.84g��cm-3)��Ũ��������1mol/L��H2SO4��Һ��100mL��
������1mol/L��NaOH��Һ������������ƽ��ȡNaOH����ʱ����ƽ����Ϊ_______(����ţ���ͬ)��
A��4.0g B����4.0g C������4.0g
������1mol/L��H2SO4��Һ�����ձ���ϡ��Ũ�������ȷ�����ǣ� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��10�֣���NaOH��������480 mL 1.0 mol/L��NaOH��Һ�������²������裺
�ٰѳ����õ�NaOH�������С�ձ��У�����������ˮ�ܽ⡣
�ڰѢ�������ҺС��ת������ƿ�С�
�ۼ���������ƿ�м�����ˮ��Һ���̶���1�M~2�M�������ý�ͷ�ι�С�ĵμ�����ˮ����Һ��Һ����̶������С�
������������ˮϴ���ձ��Ͳ�����2~3�Σ�ÿ��ϴ�ӵ�Һ�嶼С��ת������ƿ��
�ݽ�����ƿƿ�����������ҡ�ȡ�
���㡢����NaOH�����������
����д���пհף�
��1�������������ȷ˳��Ϊ������ţ� ��
��2������������������ƽ���ձ����������⣬���õ��IJ��������� ��
ʹ������ƿǰ������еIJ����� ��ʵ�����������NaOH����Ϊ g��
��3���Է������в�����������Һ��Ũ���к�Ӱ�졣���ƫ�ߡ���ƫ�͡�����Ӱ�족��
��Ϊ���ٹ����ܽ⣬�������Ȳ����Ͻ��衣��δ��������ʱ����������Һת��������ƿ���ݡ���������ҺŨ�ȵ�Ӱ�죺 ��
�ڶ��ݺӸǵ�תҡ�Ⱥ���Һ����ڿ̶��ߣ��ֵμ�����ˮ���̶ȡ���������ҺŨ�ȵ�Ӱ�죺 ��
�۶���ʱ����Һ�棬��������ҺŨ�ȵ�Ӱ�죺 ��
�ܳ�����NaOH�����л���Na2O���ʣ���������ҺŨ�ȵ�Ӱ�죺 ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��12�֣���״���£���224LHCl��������635 mLˮ�У�����������ܶ�Ϊ1.18 g��cm��3���Լ��㣺��1������������������������ʵ���Ũ���Ƕ��٣�
��2��ȡ����������100 mL��ϡ����1.18 L��������ϡ��������ʵ���Ũ�ȡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
����������������������ϡ���ᷴӦ���ų�NO��������ʵ���������
| A��FeO | B��Fe2O3 | C��FeSO4 | D��Fe3O4 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com