
”¾ĢāÄæ”æ°“ŅŖĒóĶź³ÉĻĀĮŠĢīæÕ
£Ø1£©¼ģŃéĢśĄė×Ó£ØFe3£«£©£ŗŹŌ¼Į____________£ØĢī»ÆѧŹ½£©£»ĻÖĻó______________”£ÓŠĢśĄė×Ó£ØFe3£«£©µÄ»·¾³ĻĀ¼ģŃéŹĒ·ńŗ¬ÓŠŃĒĢśĄė×Ó£ØFe2£«£©£ŗŹŌ¼Į£ŗ_________£ØĢī»ÆѧŹ½£©£¬ĻÖĻó_______________”£
£Ø2£©ÓĆŠ”ĖÕ“ņʬÖĪĮĘĪøĖį¹ż¶ąµÄĄė×Ó·½³ĢŹ½ĪŖ____________”£
£Ø3£©Ć¾“ų×Å»šŹ±£¬²»ÄÜÓĆŅŗĢ¬CO2Ćš»š¼ĮĄ“Ćš»šµÄŌŅņŹĒ_____________”££ØÓĆ»Æѧ·½³ĢŹ½±ķŹ¾£©£®
£Ø4£©³żČ„»ģČėFe2O3·ŪÄ©ÖŠÉŁĮæAl2O3ŌÓÖŹĄė×Ó·½³ĢŹ½ĪŖ______________”£
£Ø5£©½«AlCl3ČÜŅŗÕōøÉ£¬×ĘÉÕ£¬µĆµ½µÄ¹ĢĢå²śĪļŹĒ__________”£AlCl3ČÜŅŗŗĶNaHCO3ČÜŅŗ»ģŗĻŹ±·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ_________________”£
”¾“š°ø”æKSCN ČÜŅŗ±äŗģ K3[Fe(CN)6] ÓŠĄ¶É«³ĮµķÉś³É»ņKMnO4£¬×ĻŗģÉ«ĶŹČ„ HCO3££«H£«£½CO2”ü£«H2O 2Mg£«CO2 ![]() 2MgO£«C Al2O3£«2OH-£½2AlO2--£«H2O Al2O3 Al3£«£«3HCO3-£½Al£ØOH£©3”ż£«3CO2”ü
2MgO£«C Al2O3£«2OH-£½2AlO2--£«H2O Al2O3 Al3£«£«3HCO3-£½Al£ØOH£©3”ż£«3CO2”ü
”¾½āĪö”æ
£Ø1£©¼ģŃéĢśĄė×Ó³£Ń”ÓƵďŌ¼ĮĪŖĮņĒč»Æ¼ŲČÜŅŗ£»ČōČÜŅŗÖŠ“ęŌŚĢśĄė×Ó£¬æÉŅŌ¼ÓČėĢśĒč»Æ¼ŲČÜŅŗ»ņĖįŠŌøßĆĢĖį¼ŲČÜŅŗ¼ģŃ飻
£Ø2£©ÓĆŠ”ĖÕ“ņʬÖĪĮĘĪøĖį¹ż¶ąµÄ·“Ó¦ĪŖĢ¼ĖįĒāÄĘÓėŃĪĖį·“Ӧɜ³ÉĀČ»ÆÄĘ”¢¶žŃõ»ÆĢ¼ŗĶĖ®£»
£Ø3£©Ć¾ÄÜŌŚ¶žŃõ»ÆĢ¼ÖŠ¼ĢŠųČ¼ÉÕÉś³ÉŃõ»ÆĆ¾ŗĶĢ¼£»
£Ø4£©Ńõ»ÆĢśŹĒ¼īŠŌŃõ»ÆĪļ£¬²»ÄÜÓėĒāŃõ»ÆÄĘČÜŅŗ·“Ó¦£¬Ńõ»ÆĀĮĪŖĮ½ŠŌĒāŃõ»ÆĪļ£¬ÄÜÓėĒāŃõ»ÆÄĘČÜŅŗ·“Ó¦£»
£Ø5£©AlCl3ŌŚČÜŅŗÖŠĖ®½āÉś³ÉĒāŃõ»ÆĀĮŗĶĀČ»ÆĒā£¬¼ÓČČÕōøÉŹ±£¬ĀČ»ÆĒāŹÜČȻӷ¢£¬Ź¹Ė®½āĘ½ŗāĻņÓŅŅʶÆĒ÷ÓŚĶźČ«Éś³ÉĒāŃõ»ÆĀĮ£»AlCl3ŗĶNaHCO3ŌŚČÜŅŗÖŠ·¢ÉśĖ«Ė®½ā·“Ӧɜ³ÉĒāŃõ»ÆĀĮ³ĮµķŗĶ¶žŃõ»ÆĢ¼ĘųĢ唣
£Ø1£©¼ģŃéĢśĄė×Ó³£Ń”ÓƵďŌ¼ĮĪŖĮņĒč»Æ¼ŲČÜŅŗ£¬ČōČÜŅŗÖŠ“ęŌŚĢśĄė×Ó£¬ČÜŅŗ»į±ä³ÉŗģÉ«£»ČōČÜŅŗÖŠ“ęŌŚĢśĄė×Ó£¬ĪŖ·ĄÖ¹Čż¼ŪĢśøÉČżģŃ飬æÉŅŌ¼ÓČėĢśĒč»Æ¼ŲČÜŅŗ¼ģŃ飬ČōČÜŅŗÖŠÓŠŃĒĢśĄė×Ó£¬ČÜŅŗÖŠ»įÓŠĄ¶É«³ĮµķÉś³É£¬Ņ²æÉŅŌ¼ÓČėĖįŠŌøßĆĢĖį¼ŲČÜŅŗ¼ģŃ飬ČōČÜŅŗÖŠÓŠŃĒĢśĄė×Ó£¬ČÜŅŗ×ĻŗģÉ«»įĶŹČ„£¬¹Ź“š°øĪŖ£ŗKSCNČÜŅŗ£»ČÜŅŗ±äĪŖŃŖŗģÉ«£»K3[Fe(CN)6]£»ÓŠĄ¶É«³ĮµķÉś³É»ņKMnO4£¬×ĻŗģÉ«ĶŹČ„£»
£Ø2£©ÓĆŠ”ĖÕ“ņʬÖĪĮĘĪøĖį¹ż¶ąµÄ·“Ó¦ĪŖĢ¼ĖįĒāÄĘÓėŃĪĖį·“Ӧɜ³ÉĀČ»ÆÄĘ”¢¶žŃõ»ÆĢ¼ŗĶĖ®£¬·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖHCO3££«H£«£½CO2”ü£«H2O£¬¹Ź“š°øĪŖ£ŗHCO3££«H£«£½CO2”ü£«H2O£»
£Ø3£©Ć¾“ų×Å»šŹ±£¬²»ÄÜÓĆŅŗĢ¬CO2Ćš»š¼ĮĄ“Ćš»šµÄŌŅņŹĒĆ¾ÄÜŌŚ¶žŃõ»ÆĢ¼ÖŠ¼ĢŠųČ¼ÉÕÉś³ÉŃõ»ÆĆ¾ŗĶĢ¼£¬·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ2Mg£«CO2 ![]() 2MgO£«C£¬¹Ź“š°øĪŖ£ŗ2Mg£«CO2
2MgO£«C£¬¹Ź“š°øĪŖ£ŗ2Mg£«CO2 ![]() 2MgO£«C£»
2MgO£«C£»
£Ø4£©Ńõ»ÆĢśŹĒ¼īŠŌŃõ»ÆĪļ£¬²»ÄÜÓėĒāŃõ»ÆÄĘČÜŅŗ·“Ó¦£¬Ńõ»ÆĀĮĪŖĮ½ŠŌĒāŃõ»ÆĪļ£¬ÄÜÓėĒāŃõ»ÆÄĘČÜŅŗ·“Ӧɜ³ÉĘ«ĀĮĖįÄĘŗĶĖ®£¬ĖłŅŌÓĆĒāŃõ»ÆÄĘČÜŅŗ³żČ„»ģČėFe2O3·ŪÄ©ÖŠÉŁĮæAl2O3ŌÓÖŹ£¬·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖAl2O3£«2OH-£½2AlO2--£«H2O£¬¹Ź“š°øĪŖ£ŗAl2O3£«2OH-£½2AlO2--£«H2O£»
£Ø5£©AlCl3ŌŚČÜŅŗÖŠĖ®½āÉś³ÉĒāŃõ»ÆĀĮŗĶĀČ»ÆĒā£¬¼ÓČČÕōøÉŹ±£¬ĀČ»ÆĒāŹÜČȻӷ¢£¬Ź¹Ė®½āĘ½ŗāĻņÓŅŅʶÆĒ÷ÓŚĶźČ«Éś³ÉĒāŃõ»ÆĀĮ£¬ĒāŃõ»ÆĀĮ×ĘÉÕ·Ö½āÉś³ÉŃõ»ÆĀĮŗĶĖ®£¬×īÖÕµĆµ½µÄ¹ĢĢåĪŖŃõ»ÆĀĮ£»AlCl3ŗĶNaHCO3ŌŚČÜŅŗÖŠ·¢ÉśĖ«Ė®½ā·“Ӧɜ³ÉĒāŃõ»ÆĀĮ³ĮµķŗĶ¶žŃõ»ÆĢ¼ĘųĢ壬·“Ó¦Ąė×Ó·½³ĢŹ½ĪŖAl3£«£«3HCO3-£½Al£ØOH£©3”ż£«3CO2”ü£¬¹Ź“š°øĪŖ£ŗAl3£«£«3HCO3-£½Al£ØOH£©3”ż£«3CO2”ü”£



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æW”¢M”¢X”¢Y”¢ZŹĒÖÜĘŚ±ķĒ°36ŗÅŌŖĖŲÖŠµÄĖÄÖÖ³£¼ūŌŖĖŲ£¬ĘäŌ×ÓŠņŹżŅĄ“ĪŌö“ó”£WµÄŅ»ÖÖŗĖĖŲŌŚæ¼¹ÅŹ±³£ÓĆĄ“¼ų¶ØŅ»Š©ĪÄĪļµÄÄź“ś£»MµÄŃõ»ÆĪļŹĒµ¼ÖĀĖįÓźµÄÖ÷ŅŖĪļÖŹÖ®Ņ»”£XµÄijŅ»ÖÖµ„ÖŹŌŚøßæÕ“óĘų²ćÖŠ±£»¤ČĖĄąĆāŌāĢ«Ńō¹āÖŠ×ĻĶāĻßµÄĒæĮŅĒÖĻ®£»YµÄ»łĢ¬Ō×ÓŗĖĶāÓŠ6øöŌ×Ó¹ģµĄ“¦ÓŚ°ė³äĀś×“Ģ¬£»ZÄÜŠĪ³ÉŗģÉ«µÄZ2OŗĶŗŚÉ«µÄZOĮ½ÖÖŃõ»ÆĪļ”£
(1)Y3£«»łĢ¬µē×ÓÅŲ¼Ź½æɱķŹ¾ĪŖ________”£
(2)MX3£µÄæռ乹ŠĶŹĒ________(ÓĆĪÄ×ÖĆčŹö)”£H2X·Ö×ÓµÄVSEPRÄ£ŠĶĆū³ĘĪŖ________”£
(3)ŗ¬Z(H2X)42+µÄČÜŅŗÖŠĶØČėMH3£¬»įÉś³ÉZ(MH3)42+µÄŌŅņ___________________________”££Ø“ÓµēøŗŠŌ½Ē¶Č½āŹĶ£©
(4) 1 mol WX2ÖŠŗ¬ÓŠµÄ¦Š¼üŹżÄæĪŖ________”£
(5) AlPŅņɱ³ęŠ§ĀŹøß”¢Į®¼ŪŅ׵ƶų±»¹ć·ŗÓ¦ÓĆ”£ŅŃÖŖAlPµÄČŪµćĪŖ2000”ę £¬Ę侧°ū½į¹¹ČēĶ¼ĖłŹ¾”£
¢ŁĮ×»ÆĀĮµÄ¾§ĢåĄąŠĶĪŖ_____________”£
¢ŚA”¢BµćµÄŌ×Ó×ų±źČēĶ¼ĖłŹ¾£¬ŌņCµćµÄŌ×Ó×ų±źĪŖ_______”£
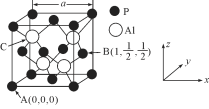
¢ŪĮ×»ÆĀĮµÄ¾§°ū²ĪŹża£½546.35pm£Ø1pm£½10-12m£©£¬ĘäĆܶČĪŖ____________ g/cm3£ØĮŠ³ö¼ĘĖćŹ½¼“æÉ£¬ÓĆNA±ķŹ¾°¢·ü¼ÓµĀĀŽ³£ŹżµÄŹżÖµ£©”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æ½ńÓŠ»ÆŗĻĪļ£ŗ
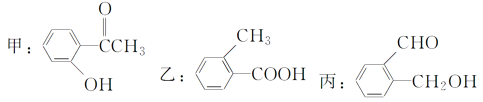
(1)ĒėŠ“³ö±ūÖŠŗ¬Ńõ¹ŁÄÜĶŵÄĆū³Ę£ŗ___________”¢__________”£
(2)ĒėÅŠ±šÉĻŹöÄÄŠ©»ÆŗĻĪļ»„ĪŖĶ¬·ÖŅģ¹¹Ģå£ŗ____”¢______”¢_____”£(ÓĆ”°¼×£¬ŅŅ£¬±ū”±±ķŹ¾)
(3)Ēė·Ö±šŠ“³ö¼ų±š¼×”¢ŅŅ”¢±ūČżÖÖ»ÆŗĻĪļµÄ·½·Ø(ÖøĆ÷ĖłŃ”ŹŌ¼Į¼°Ö÷ŅŖĻÖĻó¼“æÉ)”£
¼ų±š¼×µÄ·½·Ø£ŗŹŌ¼Į________________ĻÖĻó________________________£»
¼ų±šŅŅµÄ·½·Ø£ŗŹŌ¼Į________________ĻÖĻó________________________£»
¼ų±š±ūµÄ·½·Ø£ŗŹŌ¼Į________________ĻÖĻó________________________”£
(4)Ēė°“ĖįŠŌÓÉĒæÖĮČõµÄĖ³ŠņÅÅĮŠ¼×”¢ŅŅ”¢±ūµÄĖ³Šņ£ŗ_________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æŃõ»ÆŃĒĶ(Cu2O)ŹĒŅ»ÖÖÓĆĶ¾¹ć·ŗµÄ¹āµē²ÄĮĻ£¬Ä³¹¤³§ŅŌĮņ»ÆĶæóŹÆ(ŗ¬ CuFeS2”¢Cu2SµČ)ĪŖŌĮĻÖĘČ”Cu2OµÄ¹¤ŅÕĮ÷³ĢČēĻĀ£ŗ

³£ĪĀĻĀ¼øÖÖĪļÖŹæŖŹ¼ŠĪ³É³ĮµķÓėĶźČ«³ĮµķŹ±µÄpHČēĻĀ±ķ

(1)ĀÆĘųÖŠµÄÓŠŗ¦ĘųĢå³É·ÖŹĒ___________£¬Cu2SÓėO2·“Ó¦Ź±£¬Ńõ»Æ¼ĮÓė»¹Ō¼ĮµÄĪļÖŹµÄĮæÖ®±ČĪŖ___________”£
(2)ČōŹŌ¼ĮXŹĒH2O2ČÜŅŗ£¬Š“³öĻąÓ¦·“Ó¦µÄĄė×Ó·½³ĢŹ½£ŗ______________________”£µ±ŹŌ¼ĮXŹĒ___________Ź±£¬øüÓŠĄūÓŚ½µµĶÉś²ś³É±¾”£
(3)¼ÓČėŹŌ¼ĮYµ÷pHŹ±£¬pHµÄµ÷æŲ·¶Ī§ŹĒ______________________”£
(4)Š“³öÓĆN2H4ÖʱøCu2OµÄ»Æѧ·½³ĢŹ½£ŗ______________________£¬²Ł×÷X°üĄØ___________”¢Ļ“µÓ”¢ŗęøÉ£¬ĘäÖŠŗęøÉŹ±ŅŖøō¾ųæÕĘų£¬ĘäÄæµÄŹĒ___________”£
(5)ŅŌĶÓėŹÆÄ«×÷µē¼«£¬µē½āÅصÄĒæ¼īŠŌČÜŅŗæÉÖʵĆÄÉĆ×¼¶Cu2O£¬Š“³öŃō¼«ÉĻÉś³ÉCu2OµÄµē¼«·“Ó¦Ź½£ŗ_________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĪŖŃéÖ¤Ķ¬Ö÷×åŌŖĖŲŠŌÖŹµÄµŻ±ä¹ęĀÉ”£Ä³Š”×éÓĆČēĶ¼ĖłŹ¾µÄ×°ÖĆ½ųŠŠŹµŃé(¼Š³ÖŅĒĘ÷ŅŃĀŌČ„£¬×°ÖĆĘųĆÜŠŌŅŃ¼ģŃé)”£
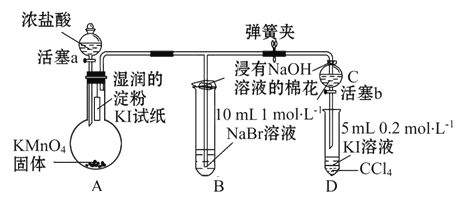
ŹµŃé¹ż³Ģ£ŗ
¢ń.“ņæŖµÆ»É¼Š£¬“ņæŖ»īČūa£¬µĪ¼ÓÅØŃĪĖį”£
¢ņ.µ±×°ÖĆBŗĶ×°ÖĆCÖŠµÄČÜŅŗ¶¼±äĪŖ»ĘÉ«Ź±£¬¼Š½ōµÆ»É¼Š”£
¢ó.µ±×°ÖĆBÖŠČÜŅŗÓÉ»ĘÉ«±äĪŖ×ŲŗģÉ«Ź±£¬¹Ų±Õ»īČūa”£
¢ō.””
£Ø1£©½žÓŠNaOHČÜŅŗµÄĆŽ»ØµÄ×÷ÓĆ____________________________”£
£Ø2£©×°ÖĆAÖŠ·¢ÉśµÄÖĆ»»·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ___________________”£
£Ø3£©×°ÖĆBµÄČÜŅŗÖŠNaBrĶźČ«±»Ńõ»Æ£¬ŌņĻūŗÄCl2µÄĪļÖŹµÄĮæĪŖ__________”£
£Ø4£©ĪŖŃéÖ¤äåŌŖĖŲµÄ·Ē½šŹōŠŌĒæÓŚµāŌŖĖŲ£¬¹ż³Ģ¢ōµÄ²Ł×÷ŗĶĻÖĻóŹĒ__________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æ¶ĢÖÜĘŚŌŖĖŲX”¢Y”¢Z”¢WŌŚŌŖĖŲÖÜĘŚ±ķÖŠµÄĻą¶ŌĪ»ÖĆČēĻĀĶ¼ĖłŹ¾£¬ĘäÖŠWŌ×ÓµÄ×īøß»ÆŗĻ¼ŪÓė×īµĶ»ÆŗĻ¼Ū“śŹżŗĶĪŖ2 £¬ĻĀĮŠĖµ·Ø²»ÕżČ·µÄŹĒ( )
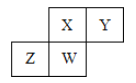
A.Ō×Ó°ė¾¶£ŗW>Z>Y>X
B.×īøß¼ŪŃõ»ÆĪļ¶ŌÓ¦Ė®»ÆĪļµÄĖįŠŌ£ŗX>W>Z
C.×ī¼ņµ„ĘųĢ¬Ēā»ÆĪļµÄČČĪČ¶ØŠŌ£ŗY>X>W>Z
D.ŌŖĖŲX”¢Z”¢WµÄ×īøß»ÆŗĻ¼Ū·Ö±šÓėĘäÖ÷×åŠņŹżĻąµČ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĻĀĶ¼ŹĒ¹¤ŅµÉĻĀĮĶĮæó(ŗ¬ÓŠAl2O3ŗĶFe2O3µČ)Ņ±Į¶ĀĮµÄ¹¤ŅÕĮ÷³ĢĶ¼£ŗ
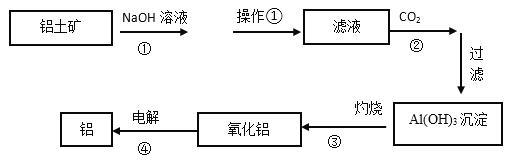
(1)²Ł×÷¢ŁµÄĆū³ĘŹĒ£ŗ_________”£²Ł×÷¢ŁŹ£ÓąµÄæóŌüÖ÷ŅŖ³É·ÖĪŖ£ŗ__________(Ģī»ÆѧŹ½)”£
(2)Ķ¼ÖŠ¢ŁµÄĄė×Ó·½³ĢŹ½___________________________£»¢ÜµÄ»Æѧ·“Ó¦·½³ĢŹ½___________”£
(3)Ļ“µÓAl(OH)3³ĮµķµÄ¾ßĢå²Ł×÷ŹĒ£ŗ ________________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æŃŠ¾æ·¢ĻÖ£¬ŌŚCO2µĶŃ¹ŗĻ³É¼×“¼·“Ó¦(CO2+3H2=CH3OH+H2O)ÖŠ£¬CoŃõ»ÆĪļøŗŌŲµÄMnŃõ»ÆĪļÄÉĆ×Į£×ӓ߻ƼĮ¾ßÓŠøß»īŠŌ£¬ĻŌŹ¾³öĮ¼ŗƵÄÓ¦ÓĆĒ°¾°”£»Ų“šĻĀĮŠĪŹĢā:
(1)Co»łĢ¬Ō×ÓŗĖĶāµē×ÓÅŲ¼Ź½ĪŖ____”£ŌŖĖŲMnÓėOÖŠ£¬µēøŗŠŌ½Ļ“óµÄŹĒ___£¬»łĢ¬Ō×ÓŗĖĶāĪ“³É¶Ōµē×ÓŹż½Ļ¶ąµÄŹĒ____”£
(2)CO2ŗĶCH3OH·Ö×ÓÖŠCŌ×ÓµÄŌӻƊĪŹ½·Ö±šĪŖ____ŗĶ____”£
(3)ŌŚCO2µĶŃ¹ŗĻ³É¼×“¼·“Ó¦ĖłÉę¼°µÄ4ÖÖĪļÖŹÖŠ£¬·Šµć“Óøßµ½µĶµÄĖ³ŠņĪŖ____”£
(4)ĻõĖįĆĢŹĒÖʱøÉĻŹö·“Ó¦“߻ƼĮµÄŌĮĻ£¬Mn(NO3)2ÖŠµÄ»Æѧ¼ü³żĮĖ¦Ņ¼üĶā£¬»¹“ęŌŚ____”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĻÖÓŠ³£ĪĀĻĀ¼×”¢ŅŅ”¢±ūČżÖÖČÜŅŗ£¬¼×ĪŖ0.1 mol”¤L£1µÄNaOHČÜŅŗ£¬ŅŅĪŖ0.1 mol”¤L£1µÄHClČÜŅŗ£¬±ūĪŖ0.1 mol”¤L£1µÄCH3COOHČÜŅŗ£¬ŹŌ»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©¼×ČÜŅŗµÄpH£½________”£
£Ø2£©±ūČÜŅŗÖŠ“ęŌŚµÄµēĄėĘ½ŗāĪŖ______________(ÓƵēĄėĘ½ŗā·½³ĢŹ½±ķŹ¾)”£
£Ø3£©³£ĪĀĻĀ£¬ÓĆĖ®Ļ”ŹĶ0.1 mol”¤L£1µÄCH3COOHČÜŅŗŹ±£¬ĻĀĮŠø÷ĮæĖęĖ®ĮæµÄŌö¼Ó¶ųŌö“óµÄŹĒ________(ĢīŠņŗÅ)”£
¢Łn(H£«)””””””””””¢Śc(H£«) ¢Ū c(CH3COOH)/c(CH3COO-) ¢Üc(OH£)
£Ø4£©¼×”¢ŅŅ”¢±ūČżÖÖČÜŅŗÖŠÓÉĖ®µēĄė³öµÄc(OH£)µÄ“󊔹ŲĻµĪŖ___________”£
£Ø5£©Ä³Ķ¬Ń§ÓĆ¼×ČÜŅŗ·Ö±šµĪ¶Ø20.00 mLŅŅČÜŅŗŗĶ20.00 mL±ūČÜŅŗ£¬µĆµ½ČēĶ¼ĖłŹ¾µÄĮ½ĢõµĪ¶ØĒśĻߣ¬Ēė»Ų“šÓŠ¹ŲĪŹĢā£ŗ
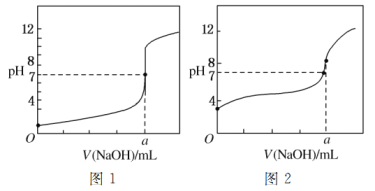
¢Ł¼×ČÜŅŗµĪ¶Ø±ūČÜŅŗµÄĒśĻߏĒ________(Ģī”°Ķ¼1”±»ņ”°Ķ¼2”±)ĒśĻß”£
¢Śa£½________”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com