
Ģ¼ŹĒŠĪ³É»ÆŗĻĪļÖÖĄą×ī¶ąµÄŌŖĖŲ£¬µŖŌŖĖŲæÉŅŌŠĪ³É¶ąÖÖ»ÆŗĻĪļ£¬ŃõŹĒµŲæĒÖŠŗ¬Įæ×ī¶ąµÄŌŖĖŲ”£
»Ų“šŅŌĻĀĪŹĢā£ŗ£Ø12·Ö£¬ĆææÕ2·Ö£©
£Ø1£©”°µĶĢ¼Éś»ī”±³«µ¼µĶÄÜĮ攢µĶĻūŗÄ£¬Ö÷ŅŖŹĒĪŖĮĖ¼õÉŁ £ØĢī»ÆѧŹ½£©µÄÅÅ·ÅĮ森
£Ø2£©»łĢ¬µŖŌ×ӵļŪµē×ÓÅŲ¼Ź½ŹĒ_________ ________”£
£Ø3£©ŃõŌŖĖŲ»łĢ¬Ō×ÓŗĖĶāĪ“³É¶Ōµē×ÓŹżĪŖ øö”£
£Ø4£©C”¢N”¢OČżÖÖŌŖĖŲµŚŅ»µēĄėÄܓӓ󵽊”µÄĖ³ŠņŹĒ____________”£
£Ø5£©ČōijŌŖĖŲEµÄŌ×Ó×īĶā²ćµē×ÓÅŲ¼ĪŖ2s22 p1£¬Ä³ŌŖĖŲFµÄŌ×ÓĶāĪ§µē×ÓÅŲ¼ĪŖ2s22p2£¬ŌņŌŖĖŲEÓėŌŖĖŲFµÄµēøŗŠŌ“ӓ󵽊”Ė³ŠņŹĒ_____ _______£ØÓĆŌŖĖŲ·ūŗűķŹ¾£©”£
ŌŖĖŲFŌŚŌŖĖŲÖÜĘŚ±ķ ĒųÓņ£ØĢīs”¢p”¢d”¢ds”¢fĒų£©
 ĆūŠ£æĪĢĆĻµĮŠ“š°ø
ĆūŠ£æĪĢĆĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
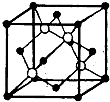 £Ø2013?ĮŁŅŹŅ»Ä££©”¾»Æѧ--ĪļÖŹ½į¹¹ÓėŠŌÖŹ”æ
£Ø2013?ĮŁŅŹŅ»Ä££©”¾»Æѧ--ĪļÖŹ½į¹¹ÓėŠŌÖŹ”æ| 160 |
| a3b |
| 160 |
| a3b |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ×ØĻīĢā ĢāŠĶ£ŗĢīæÕĢā

²éæ““š°øŗĶ½āĪö>>
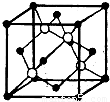
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2013ÄźÉ½¶«Ź”ĮŁŅŹŹŠøßæ¼»ÆѧŅ»Ä£ŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com