
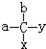 C7H16��ͬ���칹���о��С�����̼ԭ�ӡ�����2�֣�д������һ�ֵ�����3-�����飨��2��3-�������飩��
C7H16��ͬ���칹���о��С�����̼ԭ�ӡ�����2�֣�д������һ�ֵ�����3-�����飨��2��3-�������飩��
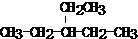
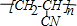 ����Ӧ�������ͷֱ�Ϊ�ӳɷ�Ӧ���Ӿ۷�Ӧ��
����Ӧ�������ͷֱ�Ϊ�ӳɷ�Ӧ���Ӿ۷�Ӧ�� ���� ��1���ٸ��л���Ϊ��������������������ԭ��Ը��л������������
�ڷ���ʽΪC7H16���л����У���������̼ԭ�ӵĸ����֪�������к�������̼ԭ�ӵĽṹ��ʽ�У�CH3CH2CH��CH3��CH2CH2CH3�ͣ�CH3��2CHCH��CH3��CH2CH3��Ȼ�������������ԭ��д������һ�ֵ����ƣ�
��2���ٸ��л�������Ϊ���飬��2��3��C������1��������4��C����1���һ����ݴ�д����ṹ��ʽ��
�������г����һ������������ٺ���5��C���ݴ�д�����л���Ľṹ��ʽ��
��4-��-3-�һ�-2-��ϩ��̼̼˫��λ��2��V������4��C���һ���3��C���ݴ�д����ṹ��ʽ��
��3������Ȳ�������ᣨHCN���ӳɷ�Ӧ��Ӧ���ɱ�ϩ�棬��ϩ�淢���Ӿ۷�Ӧ���ɾ۱�ϩ�森
��� �⣺��1��CH3CH��C2H5��CH��CH3��2�����̼������5��C����Ϊ���飬��Ŵ��ұ߿�ʼ����֧�����֮����С����2��3��C����һ���������л�������Ϊ��2��3-�������飬
�ʴ�Ϊ��2��3-�������飻
�ڸ�������̼ԭ�ӵĸ����֪��ҪʹC7H16��ͬ���칹���о��С�����̼ԭ�ӡ���������б�����һ��̼ԭ���ܹ�����4����ͬ��ԭ�ӻ����������������Ľṹ��ʽΪ��CH3CH2CH��CH3��CH2CH2CH3��CH3��2CHCH��CH3��CH2CH3����������������ԭ���֪��CH3CH2CH��CH3��CH2CH2CH3�������ǣ�3-�����飬��CH3��2CHCH��CH3��CH2CH3������Ϊ��2��3-�������飬
�ʴ�Ϊ��2��3-�����飨��2��3-�������飩��
��2����2��3-����-4-�һ����飬���л�������Ϊ���飬��2��3��C������1��������4��C����1���һ������л���Ľṹ��ʽΪ ��
��
�ʴ�Ϊ�� ��
��
�������к����һ������һ�������3��λ������ֻ��һ���һ���ʽ����С�������Ľṹ��ʽΪ��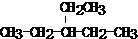 ��
��
�ʴ�Ϊ��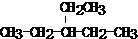 ��
��
��4-��-3-�һ�-2-��ϩ��̼̼˫����2��C����4��C����1��������3��C����1���һ������л���Ľṹ��ʽΪ��CH3CH=C��CH2CH3��CH��CH3��CH3��
�ʴ�Ϊ��CH3CH=C��CH2CH3��CH��CH3��CH3��
��4������Ȳ�������ᣨHCN����Ӧ���ɱ�ϩ��Ļ�ѧ����ʽΪ��CH��CH+HCN$\stackrel{һ��������}{��}$CH2�TCHCN���÷�ӦΪ�ӳɷ�Ӧ����ϩ�淢���Ӿ۷�Ӧ���ɾ۱�ϩ��ķ���ʽΪ��nCH2�TCHCN$\stackrel{һ������}{��}$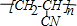 ���÷�ӦΪ�Ӿ۷�Ӧ��
���÷�ӦΪ�Ӿ۷�Ӧ��
�ʴ�Ϊ��CH��CH+HCN$\stackrel{һ��������}{��}$CH2�TCHCN��nCH2�TCHCN$\stackrel{һ������}{��}$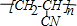 ���ӳɷ�Ӧ���Ӿ۷�Ӧ��
���ӳɷ�Ӧ���Ӿ۷�Ӧ��
���� ���⿼�����л���ṹ�����ʣ���Ŀ�Ѷ��еȣ��漰�л����������л���ṹ��ʽ��д���л���Ӧ����ʽ��д����Ӧ���͵��жϵ�֪ʶ������֪ʶ��϶࣬��ֿ�����ѧ�����Ӧ�û���֪ʶ��������ע�����ճ����л���ṹ�����ʣ���ȷ�л�������ԭ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | п��������ͭ�Ǹ��� | B�� | ���Ӵ�ͭƬ����������пƬ | ||
| C�� | �����ķ�ӦʽΪ2H++2e-=H2�� | D�� | ��Ӧһ��ʱ�����Һ��pH���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CH3CH2Br+CH3COONa��CH3COOCH2CH3+NaBr | |
| B�� | CH3I+CH3ONa��CH3OCH3+NaI | |
| C�� | CH3CH2Cl+CH3ONa��CH3Cl+CH3CH2ONa | |
| D�� | CH3CH2Cl+CH3CH2ONa����CH3CH2�� 2O+NaCl |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Ba��OH��2 | B�� | ϡ���� | C�� | ϡ���� | D�� | Ũ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | 0.01mol•L-1��CH3COOH��Һ | B�� | 0.1mol•L-1��H2SO4��Һ | ||
| C�� | pH=0��H2SO4��Һϡ100�� | D�� | c��OH-��=10-12mol•L-1����Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 920 kJ | B�� | 557 kJ | C�� | 436 kJ | D�� | 188 kJ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ŵ�ʱ������ӦΪ��3Zn-6e-+6OH-�T3Zn��OH��2 | |
| B�� | ���ʱ������ӦΪ��Fe��OH��3-3e-+5OHFeO${\;}_{4}^{2-}$+4H2O | |
| C�� | �ŵ�ʱÿת��3 mol���ӣ�������1 mol K2FeO4����ԭ | |
| D�� | ���ʱ������Һ�ļ��Լ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com