
”¾ĢāÄæ”æÅšŗĶÅšµÄ»ÆŗĻĪļŌŚ¹¤ŅµÉĻӊ׏ć·ŗµÄÓĆĶ¾”£
(1)»łĢ¬ÅšŌ×ӵĵē×ÓÅŲ¼Ź½ĪŖ___________________________”£
(2)¾§ĢåÅšµÄ»ł±¾½į¹¹µ„ŌŖŹĒÓÉÅšŌ×Ó¹¹³ÉµÄÕż¶žŹ®ĆęĢå(ČēĻĀĶ¼)£¬ĘäÖŠÓŠ20øöµČ±ßČż½ĒŠĪµÄĆęŗĶŅ»¶ØŹżÄæµÄ¶„µć£¬Ćæøö¶„µćø÷ÓŠŅ»øöÅšŌ×Ó”£“Ė½į¹¹µ„ŌŖÖŠŗ¬ÓŠÅšŌ×ӵďżÄæĪŖ________________”£
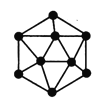
(3)µŖ»ÆÅš(BN)¾§ĢåÓŠ¶ąÖÖ½į¹¹”£Įł·½µŖ»ÆÅšÓėŹÆÄ«ĻąĖĘ£¬¾ßÓŠ²ćד½į¹¹£¬æÉ×÷øßĪĀČ󻬼Į”£Į¢·½µŖ»ÆÅšŹĒ³¬Ó²²ÄĮĻ£¬ÓŠÓÅŅģµÄÄĶÄ„ŠŌ”£ĖüĆĒµÄ¾§Ģå½į¹¹ČēĶ¼ĖłŹ¾”£¹ŲÓŚÕāĮ½ÖÖ¾§ĢåµÄĖµ·ØÖŠ£¬ÕżČ·µÄŹĒ____(Ģī±źŗÅ£©”£

a£®Į¢·½ĻąµŖ»ÆÅšŗ¬ÓŠ¦Ņ¼üŗĶ¦Š¼ü£¬ĖłŅŌÓ²¶Č“ó
b£®Įł·½ĻąµŖ»ÆÅš²ć¼ä×÷ÓĆĮ¦Š”£¬ĖłŅŌÖŹµŲČķ
c£®Į½ÖÖ¾§ĢåÖŠµÄ B”ŖN ¼ü¾łĪŖ¹²¼Ū¼ü
d£®Į½ÖÖ¾§Ģå¾łĪŖ·Ö×Ó¾§Ģå
(4)Įł·½µŖ»ÆÅš¾§ĢåÄŚÅšŌ×ÓµÄŌӻƷ½Ź½ĪŖ________£¬øĆ¾§Ģå²»µ¼µēµÄŌŅņŹĒ___________”£
(5)NH4BF4(·śÅšĖįļ§)ŹĒŗĻ³ÉµŖ»ÆÅšÄÉĆ׹ܵÄŌĮĻÖ®Ņ»”£ÓėBF4”Ŗ»„ĪŖµČµē×ÓĢåµÄŅ»ÖÖ·Ö×ӵĻÆѧŹ½ĪŖ__£¬ lmolNH4BF4¾§ĢåÖŠŗ¬ÓŠÅäĪ»¼üµÄŹżÄæĪŖ_____________”£
(6)Į¢·½µŖ»ÆÅšµÄ½į¹¹Óė½šøÕŹÆĻąĖĘ£¬Ó²¶ČÓė½šøÕŹÆĻąµ±£¬¾§°ū±ß³¤ĪŖ361.5pm£¬ŌņĮ¢·½µŖ»ÆÅšµÄŅ»øö¾§°ūÖŠŗ¬ÓŠ______øöÅšŌ×Ó£¬Į¢·½µŖ»ÆÅšµÄĆܶȏĒ____gcm£3£ØÖ»ŅŖĒóĮŠĖćŹ½£¬²»±Ų¼ĘĖć³öŹżÖµ£¬°¢·üŁ¤µĀĀŽ³£ŹżĪŖNA£©”£
”¾“š°ø”æ1s22s22p1 12 bc sp2 ²ćÓė²ćÖ®¼äĪŽ×ŌÓɵē×Ó CF4 2NA 4 ![]()
”¾½āĪö”æ
£Ø1£©øł¾ŻBµÄŌ×ÓŠņŹżĪŖ5£¬øł¾Ż¹¹ŌģŌĄķŹéŠ“ŗĖĶāµē×ÓÅŲ¼Ź½”£
£Ø2£©ÓÉĶ¼æÉÖŖ£¬ÓŠ20øöµČ±ßČż½ĒŠĪ£¬¾§ĢåÅšĆæøöČż½ĒŠĪµÄ¶„µć±»5øöČż½ĒŠĪĖł¹²ÓĆ£¬Ōņ“Ė¶„µćĶźČ«ŹōÓŚŅ»øöČż½ĒŠĪµÄÖ»Õ¼µ½1/5£¬ĆæøöČż½ĒŠĪÖŠÓŠ3øöµć£¬ĒŅ¾§ĢåÅšÖŠÓŠ20øöÕāŃłµÄČż½ĒŠĪ£¬Ōņ¾§ĢåÅšÖŠÕāŃłµÄ¶„µćÓŠ20”Į1/5”Į3=12”£
£Ø3£©¢Ła£®Į¢·½ĻąµŖ»ÆÅšÖŠNŌ×ÓÓėBŌ×ÓÖ®¼äŠĪ³Éµ„¼ü£»
b£®Įł·½ĻąµŖ»ÆÅš²ć¼ä×÷ÓĆĮ¦ĪŖ·¶µĀ»ŖĮ¦£»
c£®Į½ÖÖ¾§ĢåÖŠµÄB-N¼ü¾łĪŖ¹²¼Ū¼ü£»
d£®Į¢·½ĻąµŖ»ÆÅšŹĒ³¬Ó²²ÄĮĻ£¬ÓŠÓÅŅģµÄÄĶÄ„ŠŌ£¬ŹōÓŚŌ×Ó¾§Ģ壻
£Ø4£©Įł·½ĻąµŖ»ÆÅš¾§Ģå²ćÄŚŅ»øöÅšŌ×ÓÓėĻąĮŚ3øöµŖŌ×ÓŠĪ³É¦Ņ¼ü£»Įł·½ĻąµŖ»ÆÅš¾§ĢåµÄ²ćד½į¹¹ÖŠĆ»ÓŠ×ŌÓɵē×Ó”£
£Ø5£©¾ßÓŠĻąĶ¬¼Ūµē×Ó×ÜŹżŗĶĻąĶ¬Ō×Ó×ÜŹżµÄ·Ö×Ó»ņĄė×Ó¾ßÓŠĻąĶ¬µÄ½į¹¹ĢŲÕ÷£¬ÕāŅ»ŌĄķ³ĘĪŖ”°µČµē×ÓŌĄķ”±”£ NH4+ŗĶBF4-ø÷ŗ¬ÓŠ1øöÅäĪ»¼ü”£
£Ø6£©øł¾Ż½šøÕŹÆµÄ½į¹¹æÉŅŌÅŠ¶Ļ³ö½šøÕŹÆµÄŅ»øö¾§°ūÖŠŗ¬ÓŠµÄĢ¼Ō×ÓŹż=8”Į1/8+6”Į1/2+4=8£¬Ņņ“ĖĮ¢·½µŖ»ÆÅš¾§°ūÖŠÓ¦øĆŗ¬ÓŠ4øöNŗĶ4øöBŌ×Ó”£Ņ»øö¾§°ūµÄÖŹĮæĪŖ4”Į25g/NA£¬Ņ»øöĮ¢·½µŖ»ÆÅš¾§°ūµÄĢå»żĪŖ(361.5pm)3£¬øł¾Ż¦Ń=m/V¼ĘĖćĆܶȔ£
£Ø1£©BµÄŌ×ÓŠņŹżĪŖ5£¬»łĢ¬ÅšŌ×ӵĵē×ÓÅŲ¼Ź½ĪŖ1s22s22p1£¬¹Ź“š°øĪŖ£ŗ1s22s22p1”£
£Ø2£©ÓÉĶ¼æÉÖŖ£¬ÓŠ20øöµČ±ßČż½ĒŠĪ£¬¾§ĢåÅšĆæøöČż½ĒŠĪµÄ¶„µć±»5øöČż½ĒŠĪĖł¹²ÓĆ£¬Ōņ“Ė¶„µćĶźČ«ŹōÓŚŅ»øöČż½ĒŠĪµÄÖ»Õ¼µ½1/5£¬ĆæøöČż½ĒŠĪÖŠÓŠ3øöµć£¬ĒŅ¾§ĢåÅšÖŠÓŠ20øöÕāŃłµÄČż½ĒŠĪ£¬Ōņ¾§ĢåÅšÖŠÕāŃłµÄ¶„µćÓŠ20”Į1/5”Į3=12£¬¹Ź“š°øĪŖ£ŗ12”£
£Ø3£©a. Į¢·½ĻąµŖ»ÆÅšÖŠNŌ×ÓÓėBŌ×ÓÖ®¼äŠĪ³Éµ„¼ü£¬ĖłŅŌĪŽ¦Š¼ü£¬¹Ź“ķĪó£»
b. Įł·½ĻąµŖ»ÆÅš²ć¼ä×÷ÓĆĮ¦ĪŖ·¶µĀ»ŖĮ¦£¬Įł·½ĻąµŖ»ÆÅšÖŹµŲČķ£¬¹ŹÕżČ·£»
c.Į½ÖÖ¾§ĢåÖŠµÄB-N¼ü¾łĪŖ¹²¼Ū¼ü£¬¹ŹÕżČ·£»
d. Į¢·½ĻąµŖ»ÆÅšŹĒ³¬Ó²²ÄĮĻ£¬ÓŠÓÅŅģµÄÄĶÄ„ŠŌ£¬ŹōÓŚŌ×Ó¾§Ģ壬¹Ź“ķĪó£»
¹ŹŃ”bc”£
£Ø4£©Įł·½µŖ»ÆÅš¾§Ģå²ćÄŚŅ»øöÅšŌ×ÓÓėĻąĮŚ3øöµŖŌ×ÓŠĪ³É¦Ņ¼ü£¬ĒŅBÉĻƻӊ¹Āµē×Ó¶Ō£¬¹ŹÅšŌ×ÓµÄŌӻƷ½Ź½ĪŖsp2£»Įł·½ĻąµŖ»ÆÅš¾§ĢåµÄ²ćד½į¹¹ÖŠĆ»ÓŠ×ŌÓɵē×Ó£¬¹Ź²»Äܵ¼µē£¬¹Ź“š°øĪŖ£ŗsp2£»²ćÓė²ćÖ®¼äĪŽ×ŌÓɵē×Ó”£
£Ø5£©¾ßÓŠĻąĶ¬¼Ūµē×Ó×ÜŹżŗĶĻąĶ¬Ō×Ó×ÜŹżµÄ·Ö×Ó»ņĄė×Ó¾ßÓŠĻąĶ¬µÄ½į¹¹ĢŲÕ÷£¬ÕāŅ»ŌĄķ³ĘĪŖ”°µČµē×ÓŌĄķ”±”£ÓėBF4”Ŗ»„ĪŖµČµē×ÓĢåµÄŅ»ÖÖ·Ö×ӵĻÆѧŹ½ĪŖCF4£¬NH4BF4ÖŠNH4+ŗĶBF4-ø÷ŗ¬ÓŠ1øöÅäĪ»¼ü£¬Ōņ1molNH4BF4ÖŠŗ¬ÓŠµÄÅäĪ»¼üøöŹżĪŖ2NA£¬¹Ź“š°øĪŖ£ŗCF4£»2NA”£
£Ø6£©øł¾Ż½šøÕŹÆµÄ½į¹¹æÉŅŌÅŠ¶Ļ³ö½šøÕŹÆµÄŅ»øö¾§°ūÖŠŗ¬ÓŠµÄĢ¼Ō×ÓŹż=8”Į1/8+6”Į1/2+4=8£¬Ņņ“ĖĮ¢·½µŖ»ÆÅš¾§°ūÖŠÓ¦øĆŗ¬ÓŠ4øöNŗĶ4øöBŌ×Ó”£Ņ»øö¾§°ūµÄÖŹĮæĪŖ4”Į25g/NA£¬Ņ»øöĮ¢·½µŖ»ÆÅš¾§°ūµÄĢå»żĪŖ(361.5pm)3£¬øł¾Ż¦Ń=m/V¼ĘĖćĆÜ¶Č£¬Ņņ“ĖĮ¢·½µŖ»ÆÅšµÄĆܶȏĒ![]()
![]() gcm-3£¬¹Ź“š°øĪŖ£ŗ4£»
gcm-3£¬¹Ź“š°øĪŖ£ŗ4£»![]() ”£
ӣ


 ŌĶĮæģ³µĻµĮŠ“š°ø
ŌĶĮæģ³µĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æÓÉŅŅĻ©ĶĘ²ā±ūĻ©µÄ½į¹¹»ņŠŌÖŹ£¬ÕżČ·µÄŹĒ(””””)
A.·Ö×ÓÖŠ3øöĢ¼Ō×ÓŌŚĶ¬Ņ»Ö±ĻßÉĻ
B.·Ö×ÓÖŠĖłÓŠŌ×Ó¶¼ŌŚĶ¬Ņ»Ę½ĆęÉĻ
C.·Ö×ÓÖŠ¹²¼Ū¼üµÄ¼Š½Ē¾łĪŖ120”ć
D.·Ö×ÓÖŠ¹²¼Ū¼üŹżĪŖ8£¬ĘäÖŠÓŠŅ»øöĢ¼Ģ¼Ė«¼ü
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æA£¬B£¬C£¬DĖÄÖÖ¶ĢÖÜĘŚŌŖĖŲµÄŌ×Ó°ė¾¶ŅĄ“Ī¼õŠ”£¬DÄÜ·Ö±šÓėA£¬B£¬CŠĪ³Éµē×Ó×ÜŹżĻąµČµÄ·Ö×ÓX”¢Y”¢Z”£CŌ×ÓµÄ×īĶā²ćµē×ÓÅŲ¼ĪŖnsnnp2n”£EµÄŌ×ÓŠņŹżĪŖ29”£
(1)A£¬B£¬CµÄµŚŅ»µēĄėÄÜÓÉŠ”µ½“óµÄĖ³ŠņĪŖ________(ÓĆŌŖĖŲ·ūŗűķŹ¾)”£
(2)XŹĒŗ¬ÓŠ________¼ü(Ģī”°·Ē¼«ŠŌ”±»ņ”°¼«ŠŌ”±£¬ĻĀĶ¬)µÄ________·Ö×Ó”£
(3)AµÄŅ»ÖÖĒā»ÆĪļµÄĻą¶Ō·Ö×ÓÖŹĮæĪŖ26£¬Ęä·Ö×ÓÖŠµÄ¦Ņ¼üÓė¦Š¼üµÄ¼üŹżÖ®±ČĪŖ________”£
(4)Y·Ö×ÓµÄæռ乹ŠĶĪŖ__________£¬ĘäÖŠŠÄŌ×Ó²ÉČ”________ŌӻƔ£
(5)Ņ»ÖÖÓÉB£¬C×é³ÉµÄ»ÆŗĻĪļÓėAC2»„ĪŖµČµē×ÓĢ壬Ęä»ÆѧŹ½ĪŖ________”£
(6)YŹĒŅ»ÖÖŅ×Ņŗ»ÆµÄĘųĢ壬Ēė¼ņŹöĘäŅ×Ņŗ»ÆµÄŌŅņ_________”£
(7)Š“³öE2£«µÄµē×ÓÅŲ¼Ź½___________________£¬²¢Š“³öE2£«ŌŚZÖŠĶØČė×ćĮæYµĆµ½ÉīĄ¶É«ČÜŅŗµÄĄė×Ó·“Ó¦·½³ĢŹ½_______”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æA”¢B”¢CŹĒ֊ѧ»Æѧ³£¼ūµÄČżÖÖĪļÖŹ£¬ĖüĆĒÖ®¼äµÄĻą»„×Ŗ»Æ¹ŲĻµČēĶ¼ĖłŹ¾£Ø²æ·Ö·“Ó¦Ģõ¼ž¼°²śĪļĀŌČ„£©”£
![]()
£Ø1£©ČōAŹĒŅ»ÖÖ»īĘĆ½šŹō£¬CŹĒµ»ĘÉ«¹ĢĢ壬ŌņCµÄĆū³ĘĪŖ________________£¬CæÉÓĆŌŚæóɽ”¢æÓµĄ”¢Ē±Ė®»ņÓīÖę·É“¬µČȱŃõµÄ³”ŗĻ£¬½«ČĖĆĒŗō³öµÄCO2ŌŁ×Ŗ»»³ÉO2£¬ŅŌ¹©ŗōĪüÖ®ÓĆ£¬ĒėÓĆ»Æѧ·½³ĢŹ½±ķŹ¾øĆ·“Ó¦ŌĄķ______________________”£
£Ø2£©ČōAŹĒŅ»ÖÖ»ĘÉ«µ„ÖŹ¹ĢĢ壬ŌņB”śCµÄ»Æѧ·½³ĢŹ½ĪŖ________________”£
£Ø3£©ČōAĪŖÄÜŹ¹ŹŖČóµÄŗģÉ«ŹÆČļŹŌÖ½±äĄ¶µÄĘųĢ壬CŹĒŗģ×ŲÉ«ĘųĢ壬ĒŅBŗĶCŅ×ŌŚæÕĘųŠĪ³É¹ā»ÆѧŃĢĪķ”£
¢ŁŌņAĪŖ________________(ĢīŠ“»ÆѧŹ½£¬ĻĀĶ¬)£¬CĪŖ___________________£»ŹµŃéŹŅÓĆŹģŹÆ»ŅÓėĀČ»Æļ§¹ĢĢå¹²ČČÖĘČ”A£¬Š“³ö“Ė»Æѧ·½³ĢŹ½£ŗ_____________________________”£
¢ŚŠ“³öCÓėĖ®·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ____________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĻĀĮŠ±ķŹ¾ĪļÖŹ½į¹¹µÄ»ÆѧÓĆÓļÕżČ·µÄŹĒ(””””)
A. ŅŅĻ©µÄ½į¹¹¼ņŹ½£ŗCH2CH2 B. Ņģ±ū»łµÄ½į¹¹¼ņŹ½£ŗ-CH(CH3)2
C. ōĒ»łµÄµē×ÓŹ½£ŗ ![]() D. ŠĀĪģĶéµÄ½į¹¹¼ņŹ½£ŗ
D. ŠĀĪģĶéµÄ½į¹¹¼ņŹ½£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”潚ŹōīāŌŚ¹¤ŅµŗĶ¹ś·Ą½ØÉčÖŠÓŠÖŲŅŖµÄ×÷ÓĆ”£īā(Mo)µÄ³£¼ū»ÆŗĻ¼ŪĪŖ+6”¢+5”¢+4”£ÓÉīā¾«æó(Ö÷ŅŖ³É·ÖŹĒMoS2)æÉÖʱøµ„ÖŹīāŗĶīāĖįÄĘ¾§Ģå(Na2MoO4”¤2H2O)£¬²æ·ÖĮ÷³ĢČēĶ¼1ĖłŹ¾£ŗ
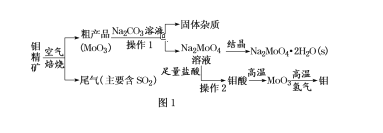
ŅŃÖŖ£ŗīāĖįĪ¢ČÜÓŚĖ®£¬æÉČÜÓŚŅŗ¼īŗĶ°±Ė®”£»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©īā¾«æó±ŗÉÕŹ±£¬ĆæÓŠ1molMoS2·“Ó¦£¬×ŖŅʵē×ÓµÄĪļÖŹµÄĮæĪŖ______”£
£Ø2£©īā¾«æó±ŗÉÕŹ±ÅŷŵÄĪ²Ęų¶Ō»·¾³µÄÖ÷ŅŖĪ£ŗ¦ŹĒ________£¬ĒėÄćÉč¼Ę¹¤ŅµÉĻ³żČ„øĆĪ²ĘųµÄ·½·Ø£ØŠ“³öĮ½ÖÖ”°±ä·ĻĪŖ±¦”±µÄ·½·ØŗĶĄė×Ó·½³ĢŹ½£©£ŗ___________£»___________”£
£Ø3£©ÓÉīāĖįµĆµ½MoO3ĖłÓƵ½µÄ¹čĖįŃĪ²ÄĮĻŅĒĘ÷µÄĆū³ĘŹĒ_______”£
£Ø4£©²Ł×÷1ÖŠ£¬¼ÓČėĢ¼ĖįÄĘČÜŅŗ³ä·Ö·“Ó¦ŗ󣬼ī½žŅŗÖŠc(MoO42-)£½0.80mol/L£¬c(SO42-)£½0.04mol/L£¬ŌŚ½į¾§Ē°Šč¼ÓČėBa(OH)2¹ĢĢåŅŌ³żČ„ČÜŅŗÖŠµÄSO42-”£µ±BaMoO4æŖŹ¼³ĮµķŹ±£¬SO42-µÄČ„³żĀŹŹĒ_____”£[Ksp(BaSO4)£½1.1”Į1010”¢Ksp(BaMoO4)£½4.0”Į108£¬ČÜŅŗĢå»ż±ä»ÆæÉŗöĀŌ²»¼Ę]
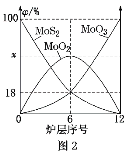
£Ø5£©±ŗÉÕīā¾«æóĖłÓƵÄ×°ÖĆŹĒ¶ą²ć±ŗÉÕĀÆ£¬Ķ¼2ĪŖø÷ĀƲć¹ĢĢåĪļĮĻµÄĪļÖŹµÄĮæµÄ°Ł·ÖŹż(¦Õ)”£
¢Łx£½_____”£
¢Ś±ŗÉÕĀÆÖŠŅ²»į·¢ÉśMoS2ÓėMoO3·“Ӧɜ³ÉMoO2ŗĶSO2µÄ·“Ó¦£¬ČōøĆ·“Ó¦×ŖŅĘ6molµē×Ó£¬ŌņĻūŗĵÄŃõ»Æ¼ĮµÄĪļÖŹµÄĮæĪŖ____”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æ20”ꏱ£¬ŌŚH2C2O4”¢NaOH»ģŗĻČÜŅŗÖŠ£¬c£ØH2C2O4£©£«c£ØHC2O4-£©£«c£ØC2O42-£©£½0.100mol/L”£ŗ¬Ģ¼ŌŖĖŲĪ¢Į£µÄ·Ö²¼·ÖŹż¦ÄĖęČÜŅŗpH±ä»ÆµÄ¹ŲĻµČēĶ¼ĖłŹ¾”£ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ£Ø £©
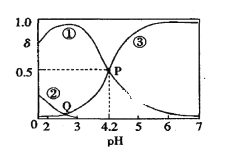
A. ¢Ł±ķŹ¾H2C2O4µÄ·Ö²¼ĒśĻߣ¬¢Ū±ķŹ¾C2O42-µÄ·Ö²¼ĒśĻß
B. 20”ꏱ£¬H2C2O4µÄ¶ž¼¶µēĄėĘ½ŗā³£ŹżKa2£½1”Į10£4.2
C. Qµć¶ŌÓ¦ČÜŅŗÖŠlgc£ØH£«£©£¼lgc£ØOH££©
D. 0.100mol”¤L£1µÄNaHC2O4ČÜŅŗÖŠ£ŗc£ØOH££©£½c£ØH£«£©£2c£ØC2O42-£©£«c£ØH2C2O4£©
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æÓĆĻĀĮŠŹµŃé×°ÖĆ½ųŠŠĻąÓ¦ŹµŃ飬ÄÜ“ļµ½ŹµŃéÄæµÄµÄŹĒ

A.ÓĆĶ¼1ĖłŹ¾×°ÖĆ³żČ„Cl2ÖŠŗ¬ÓŠµÄÉŁĮæHCl
B.ÓĆĶ¼2ĖłŹ¾×°ÖĆÕōøÉNH4Cl±„ŗĶČÜŅŗÖʱøNH4Cl¾§Ģå
C.ÓĆĶ¼3ĖłŹ¾×°ÖĆÖĘȔɣĮæ“æ¾»µÄCO2ĘųĢå
D.ÓĆĶ¼4ĖłŹ¾×°ÖĆ·ÖĄėCCl4ŻĶČ”µāĖ®ŗóŅŃ·Ö²ćµÄÓŠ»ś²ćŗĶĖ®²ć
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æ³£ĪĀĻĀĻņ20mL 0.1mol/L°±Ė®ÖŠĶØČėHClĘųĢ壬ČÜŅŗÖŠÓÉĖ®µēĄė³öµÄĒāĄė×ÓÅضČĖęĶØČėHClĘųĢåµÄĢå»ż±ä»ÆČēĶ¼ĖłŹ¾”£ŌņĻĀĮŠĖµ·ØÕżČ·µÄŹĒ
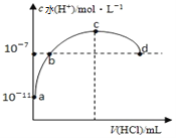
A. bµćĶØČėµÄHClĘųĢ壬ŌŚ±źæöĻĀĪŖ44.8mL
B. b”¢cÖ®¼äČÜŅŗÖŠc(NH4+)>c(Cl-)
C. Č”10mLµÄcµćČÜŅŗĻ”ŹĶŹ±£ŗc(NH4+)/c(NH3”¤H2O)¼õŠ”
D. dµćČÜŅŗ³ŹÖŠŠŌ
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com