
| A£® | ²»ÄÜÅŠ¶ĻČÜŅŗÖŠŹĒ·ń“ęŌŚSO42- | |
| B£® | ČÜŅŗÖŠŅ»¶Ø²»“ęŌŚµÄĄė×ÓŹĒCO${\;}_{3}^{2-}$ | |
| C£® | ²»ÄÜÅŠ¶ĻČÜŅŗÖŠŹĒ·ń“ęŌŚAg+ | |
| D£® | ²»ÄÜÅŠ¶ĻŹĒ·ńŗ¬ÓŠ AlO2- Ąė×Ó |
·ÖĪö ČÜŅŗĪŽÉ«£¬Ņ»¶ØƻӊøßĆĢĖįøłĄė×Ó£¬
¢ŁÄܹ»ÓėŃĪĖįÉś³ÉĘųĢåµÄĄė×ÓĪŖĢ¼ĖįøłĄė×Ó£¬Äܹ»ÓėĢ¼ĖįøłĄė×Ó·“Ó¦µÄĄė×Ó²»ÄÜ“ęŌŚ£»
¢ŚĖµĆ÷·¢ÉśĮĖĖ«Ė®½ā£¬Ņ»¶Ø“ęŌŚÓėĢ¼ĖįĒāøłĄė×Ó·¢ÉśĖ«Ė®½āµÄĄė×Ó£»
¢ŪĘųĢåĪŖ°±Ęų£¬°×É«³ĮµķĪŖĢ¼Ėį±µ»ņĮņĖį±µ£¬¾Ż“Ė½ųŠŠĶʶĻ£®
½ā“š ½ā£ŗijĪŽÉ«ČÜŅŗ£¬ĖµĆ÷ČÜŅŗÖŠŅ»¶Ø²»»į“ęŌŚøßĆĢĖįøłĄė×Ó£¬
¢Ł¼ÓČė¹żĮæŃĪĖį£¬ÓŠĘųĢåÉś³É£¬²¢µĆµ½ĪŽÉ«ČÜŅŗ£¬Éś³ÉµÄĘųĢåĪŖ¶žŃõ»ÆĢ¼£¬ĖłŅŌČÜŅŗÖŠŅ»¶Ø“ęŌŚCO32-£¬Ņ»¶Ø²»“ęŌŚAg+”¢Ba2+”¢Al3+£¬ŃōĄė×ÓÖ»Ź£ĻĀĮĖÄĘĄė×Ó£¬øł¾ŻČÜŅŗŅ»¶Ø³ŹµēÖŠŠŌæÉÖŖČÜŅŗÖŠŅ»¶Ø“ęŌŚNa+£»
¢ŚŌŚ¢ŁĖłµĆČÜŅŗÖŠ¼ÓČė¹żĮæNH4HCO3ČÜŅŗ£¬ÓŠĘųĢåÉś³É£¬Ķ¬Ź±Īö³ö°×É«³Įµķ¼×£¬°×É«³Įµķ¼×ĪŖĒāŃõ»ÆĀĮ£¬ŌČÜŅŗÖŠŅ»¶Ø“ęŌŚAlO2-£¬
¢ŪŌŚ¢ŚĖłµĆČÜŅŗÖŠ¼ÓČė¹żĮæBa£ØOH£©2ČÜŅŗ£¬Ņ²ÓŠĘųĢåÉś³É£¬Ķ¬Ź±Īö³ö°×É«³ĮµķŅŅ£¬°×É«³ĮµķŅ»¶Øŗ¬ÓŠĢ¼Ėį±µ£¬æÉÄÜŗ¬ÓŠĮņĖį±µ£»
ĖłŅŌČÜŅŗÖŠŅ»¶Ø“ęŌŚµÄĄė×ÓÓŠ£ŗCO32-”¢Na+”¢AlO2-£¬ŅųĄė×ÓŅ»¶Ø²»“ęŌŚ£»
¹ŹŃ”A£®
µćĘĄ ±¾Ģāæ¼²éĮĖ³£¼ūĄė×ӵļģŃé·½·Ø£¬ĢāÄæÄŃ¶Č²»“ó£¬×¢Ņāøł¾ŻČÜŅŗ³ŹµēÖŠŠŌÅŠ¶ĻČÜŅŗÖŠ“ęŌŚµÄĄė×Ó·½·Ø£¬±¾Ģā³ä·Öæ¼²éĮĖѧɜµÄ·ÖĪö”¢Ąķ½āÄÜĮ¦£¬ŅŖĒóŹģĮ·ÕĘĪÕ³£¼ūĄė×ӵļģŃé·½·Ø£®


 A¼Ó½šĢā ĻµĮŠ“š°ø
A¼Ó½šĢā ĻµĮŠ“š°ø Č«ÓŲāŹŌ¾ķĻµĮŠ“š°ø
Č«ÓŲāŹŌ¾ķĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
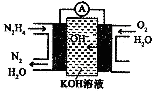 ėĀ£ØN2H4£©ÓÖ³ĘĮŖ°±£¬¹ć·ŗÓĆÓŚ»š¼żĶĘ½ų¼Į”¢»Æ¹¤ŌĮĻ¼°Č¼ĮĻµē³ŲµČ·½Ćę£®Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
ėĀ£ØN2H4£©ÓÖ³ĘĮŖ°±£¬¹ć·ŗÓĆÓŚ»š¼żĶĘ½ų¼Į”¢»Æ¹¤ŌĮĻ¼°Č¼ĮĻµē³ŲµČ·½Ćę£®Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ| »Æѧ¼ü | O-H | N-N | N-H | O=O | NØTN |
| ¼üÄÜ/KJ•mol-1 | 467 | 160 | 391 | 498 | 945 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
| 0.01mol•L-1KIO3 ĖįŠŌČÜŅŗ£Øŗ¬µķ·Ū£© µÄĢå»ż/mL | 0.01mol•L-Na2SO3 ČÜŅŗµÄĢå»ż/mL | H2OµÄ Ģå»ż/mL | ŹµŃé ĪĀ¶Č /”ę | ČÜŅŗ³öĻÖ Ą¶É«Ź±Ėł ŠčŹ±¼ä/s | |
| ŹµŃé1 | 5 | V1 | 35 | 25 | t1 |
| ŹµŃé2 | 5 | 5 | 40 | 25 | t2 |
| ŹµŃé3 | 5 | 5 | V2 | 0 | t3 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
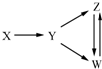 Ņ»¶ØĢõ¼žĻĀ£¬ĻĀĮŠø÷×éĪļÖŹÄÜŅ»²½ŹµĻÖĶ¼ĖłŹ¾×Ŗ»Æ¹ŲĻµµÄŹĒ£Ø””””£©
Ņ»¶ØĢõ¼žĻĀ£¬ĻĀĮŠø÷×éĪļÖŹÄÜŅ»²½ŹµĻÖĶ¼ĖłŹ¾×Ŗ»Æ¹ŲĻµµÄŹĒ£Ø””””£©| Ń”Ļī | X | Y | Z | W |
| A | Al | Al2O3 | NaAlO2 | Al£ØOH£©3 |
| B | Fe3O4 | Fe | FeCl2 | FeCl3 |
| C | H2SO4 | SO2 | S | SO3 |
| D | CH3CH2Br | CH2ØTCH2 | C2H5OH | CH2BrCH2Br |
| A£® | A | B£® | B | C£® | C | D£® | D |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
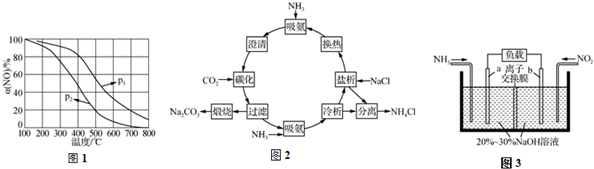
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | a”¢bŹŌ¹ÜÖŠŅŗĢå²»·Ö²ć£¬ČÜŅŗ¾łĪŖĪŽÉ« | |
| B£® | c”¢dŹŌ¹ÜÖŠŅŗĢå·Ö²ć£¬cŹŌ¹ÜÖŠÓŠ»ś²ćŌŚĻĀ£¬³Źŗģ×ŲÉ«£»dŹŌ¹ÜÖŠÓŠ»ś²ćŌŚÉĻ£¬³Źŗģ×ŲÉ« | |
| C£® | aŹŌ¹ÜÖŠÓŠ»ś²ćŌŚĻĀ£¬ĪŖĪŽÉ«£¬Ė®²ćŌŚÉĻ£¬ĪŖĪŽÉ«£»dŹŌ¹ÜÖŠÓŠ»ś²ćŌŚĻĀ£¬³Źŗģ×ŲÉ« | |
| D£® | ŅŌÉĻĆčŹö¾ł²»ÕżČ· |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ĻõĖįŅųČÜŅŗ | B£® | ŹÆČļŹŌŅŗ | ||
| C£® | ŗ¬·ÓĢŖµÄĒāŃõ»ÆÄĘČÜŅŗ | D£® | µķ·Ūµā»Æ¼ŲŹŌÖ½ |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com