
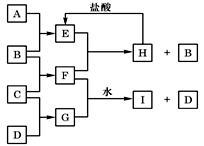
 A��B��C��D�ǰ�ԭ��������С�������еĵڶ���������Ԫ�صĵ��ʡ�B��E��Ϊ��ɿ����ijɷ֡�F����ɫ��Ӧ�ʻ�ɫ����G�У��ǽ���Ԫ�������Ԫ�ص�ԭ�Ӹ�����Ϊ1��2����һ�������£�������֮����ת����ϵ���ң�ͼ�в��ֲ���δ�г�����
A��B��C��D�ǰ�ԭ��������С�������еĵڶ���������Ԫ�صĵ��ʡ�B��E��Ϊ��ɿ����ijɷ֡�F����ɫ��Ӧ�ʻ�ɫ����G�У��ǽ���Ԫ�������Ԫ�ص�ԭ�Ӹ�����Ϊ1��2����һ�������£�������֮����ת����ϵ���ң�ͼ�в��ֲ���δ�г�����
![]() ����д���пհף�
����д���пհף�
![]() ��1��A�� ��C�� ��
��1��A�� ��C�� ��
![]() ��2��H�����ᷴӦ����E�Ļ�ѧ����ʽ�� ��
��2��H�����ᷴӦ����E�Ļ�ѧ����ʽ�� ��
![]() ��3��E��F��Ӧ�Ļ�ѧ����ʽ�� ��
��3��E��F��Ӧ�Ļ�ѧ����ʽ�� ��
![]() ��4��F��G��ˮ��Һ��Ӧ����I��D�����ӷ���ʽ�� ��
��4��F��G��ˮ��Һ��Ӧ����I��D�����ӷ���ʽ�� ��
��1��A��̼(��C) C����(��Na)����2��Na2CO3+2HCl![]() 2NaCl+H2O+CO2��
2NaCl+H2O+CO2��
![]() ��3��2CO2+2Na2O2
��3��2CO2+2Na2O2![]() 2Na2CO3+O2����4��Na2O2+S2-+2H2O
2Na2CO3+O2����4��Na2O2+S2-+2H2O![]() 4OH-+S��+2Na+
4OH-+S��+2Na+
F����ɫ��Ӧ�ʻ�ɫ��˵��F���ƵĻ������F�ǵ���B��C���϶��ɣ�B��E��Ϊ��ɿ����ijɷ֣���CΪ�����ƣ�G�ǵ���C��D���϶��ɣ�ԭ�Ӹ�����ΪD�� C��1��2����ԭ������D��C����DΪ��BΪ��ɿ����ijɷ֣��������뵽����O2����BΪO2��EҲΪ��ɿ����ijɷ֣�Eֻ����CO2��A��Ϊ̼��F��Na2O2����Na2O����ϵ��F+E��H+B����F��Na2O2��H��Na2CO3��


 ���⿼����Ԫ���Ծ�ϵ�д�
���⿼����Ԫ���Ծ�ϵ�д� ��У���˳�̾�ϵ�д�
��У���˳�̾�ϵ�д� ��У���һ��ͨϵ�д�
��У���һ��ͨϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||
| ||
| ||
| ||
| n |
| 33.6 |
| n |
| 33.6 |
| m |
| 11.2 |
| m |
| 11.2 |
| m-n |
| 22.4 |
| m-n |
| 22.4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�����ʡ�人��������������µ��в������ۻ�ѧ���� ���ͣ������
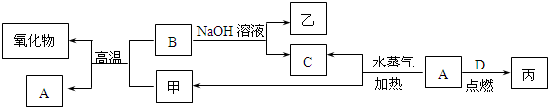
��15�֣�A��B��C��D�����ֳ����ĵ��ʣ�A��BΪ������C��D�����������壬����DΪ����ɫ���ס��ҡ���Ϊ�����Ļ��������֮���ת����ϵ����ͼ��ʾ��
�ش��������⣺
��1���������ҵ����ƣߣߣߣߣߣߣߣߣߣߡ�
��2��B���Ӧ�Ļ�ѧ����ʽ�ǣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
��3���ɱ��ı�����Һ�����Ƶý��壬����������ֱ���Ĵ�С��Χ�ǣߣߣߣߣߣߣߣߣߣߣ���Ҫ�ᴿ�ý��壬���õIJ��������Уߣߣߣߣߡ�����øý�������Ӿʵ�飬�ߣߣ�������ɫ���
��4����A��B���ֽ�����һ������������ɻ���
��ȡһ�������ĸû��������м���������NaOH��Һ���������������ڱ�״����ΪnL���������B�����ʵ���Ϊ�ߣߣߣߣ�mol���ú���ĸ�Ĵ���ʽ��ʾ����
����ȡ��ͬ�����ĸû��������м���������ϡ���ᣬ����ȫ���ܽ⣬�������������ı�״����ΪmL���÷�Ӧ��ת�Ƶ��ӵ����ʵ���Ϊ�ߣߣߣߣ�mol��ԭ�������A������Ϊ�ߣߣߣߣߣߣߣߣߣ�g���ú���ĸ�Ĵ���ʽ��ʾ����
��������õ���Һ�м������������������Һ����ֽ�����ˣ�������ϴ�ӡ�������պ�õ�һ�ֹ��壬���������ָù����������ԭ����������ǡ����ȣ���ԭ�������B����������Ϊ�ߣߣߣߣߡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ�����ʡ�����и�����һ��ģ�⿼�����ۻ�ѧ�Ծ��������棩 ���ͣ������
��֪A��B��C��D��E��F���ֶ�����Ԫ���У�A��B��C��D����ɵ����ʵĻ���Ԫ�أ�A��B��ԭ������֮�͵���Cԭ�Ӻ��ڵ���������A��E��D��F�ֱ�λ��ͬһ���壬��Fԭ�Ӻ��ڵ���������Dԭ�Ӻ����������2�����ݴˣ���ش�
��1��F�����ڱ��е�λ����____________________________��
��2����A��C��D��F��8:2:4:1ԭ�Ӹ�������ɵĻ�������к��еĻ�ѧ������Ϊ____________������Һ�и�����Ũ���ɴ�С��˳��Ϊ________________��������Ũ�ȷ��ű�ʾ����
��3������������A��C�������Է�������Ϊ32�����������A��D����ҷ����ڵ����������ҷ����ڵ���������ȣ�������ķ�Ӧ�����ڻ�����䣨��Ӧ���ﲻ��Ⱦ����������÷�Ӧ�Ļ�ѧ����ʽΪ_________________________________________��
��4����A��D��E��F��ɵĻ����ﶡ�������ᷴӦ���ų��̼�����ζ�����壬�Ļ�ѧʽΪ�ߣߣߣߣߣߣߣߣ�ʵ���ö���Һ�������ԣ��ɴ����ܵó��Ľ�����___________________��
��5����B��A��1:4ԭ�Ӹ�������ɵĻ���������D�ij�����̬���ʼ�NaOH��Һ����ԭ��� ����ͼ�����Է�����
����ͼ�����Է�����

���պ�K��д�����X�缫�ķ�Ӧʽ__________________________________��
���պ�K����X�缫����1.6g��������ʱ��������������κ���ʧ�������ҳ��������ų������ڱ�״���µ����Ϊ_________����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��B��C��D�����ֳ����ĵ��ʣ�A��BΪ������C��D�����������壬����DΪ����ɫ���ס��ҡ���Ϊ�����Ļ��������֮���ת����ϵ����ͼ��ʾ��

�ش��������⣺
��1���������ҵ����ƣߣߣߣߣߣߣߣߣߣߡ�
��2��B���Ӧ�Ļ�ѧ����ʽ�ǣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
��3���ɱ��ı�����Һ�����Ƶý��壬����������ֱ���Ĵ�С��Χ�ǣߣߣߣߣߣߣߣߣߣߣ���Ҫ�ᴿ�ý��壬���õIJ��������Уߣߣߣߣߡ�����øý�������Ӿʵ�飬�ߣߣ�������ɫ���
��4����A��B���ֽ�����һ������������ɻ���
��ȡһ�������ĸû��������м���������NaOH��Һ���������������ڱ�״����ΪnL���������B�����ʵ���Ϊ�ߣߣߣߣ�mol���ú���ĸ�Ĵ���ʽ��ʾ����
����ȡ��ͬ�����ĸû��������м���������ϡ���ᣬ����ȫ���ܽ⣬�������������ı�״����ΪmL���÷�Ӧ��ת�Ƶ��ӵ����ʵ���Ϊ�ߣߣߣߣ�mol��ԭ�������A������Ϊ�ߣߣߣߣߣߣߣߣߣ�g���ú���ĸ�Ĵ���ʽ��ʾ����
��������õ���Һ�м������������������Һ����ֽ�����ˣ�������ϴ�ӡ�������պ�õ�һ�ֹ��壬���������ָù����������ԭ����������ǡ����ȣ���ԭ�������B����������Ϊ�ߣߣߣߣߡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��B��C��D�����ֳ����ĵ��ʣ�A��BΪ������C��D�����������壬��DΪ����ɫ���塣�ס��ҡ���Ϊ�����Ļ�������Ǻ�ɫ�Ҿ��д��Ե����ʡ�����֮���ת����ϵ����ͼ��ʾ��

��ش��������⣺
��1��B���Ӧ�Ļ�ѧ����ʽ�� ��
��2�������£���A��B�ĵ��ʷ���Ũ�����Ũ�����У��Ƿ��ܽ⣿ ����ǡ�����
��3����������ˮ�����Һ��������������ӵķ�����
��
��4��д��A��ˮ������Ӧ����C�ͼĻ�ѧ����ʽ ��
��5����A��B���ֽ�����һ������������ɻ���
��ȡһ�������ĸû��������м���������NaOH��Һ���������������ڱ�״����Ϊn L��B��NaOH��Һ��Ӧ�����ӷ���ʽ�� ���������B�����ʵ���Ϊ mol���ú���ĸ�ķ���ʽ��ʾ����
����ȡ��ͬ�����ĸû��������м���������ϡ���ᣬ����ȫ���ܽ⣬�������������ڱ�״����Ϊm L���÷�Ӧ��ת�Ƶ��ӵ����ʵ���Ϊ mol���������A������Ϊ g���ú���ĸ�ķ���ʽ��ʾ����
��������õ���Һ�м������������������Һ����ֽ��裬�������ij������˳�������ϴ�ӡ�������պ�õ�һ�ֹ��壬���������ָù����������ԭ����������ǡ����ȣ���ԭ�������������������Ϊ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com