
��10�֣���ͼ�����ڼ��л���������Ʊ������롢���ʱȽϵȵij�������װ�á���
���ݸ�װ�ûش��������⣺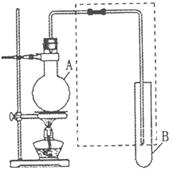
��1�������Ҵ������ᷴӦ��ȡ��������������ƿA
�м�����Լ��ڻ��ʱ�IJ���������___________����
Ӧ�Ļ�ѧ����ʽΪ___________ ���Թ�B��Ӧ
����___________ �����߿��еĵ��ܳ����ڵ���
�⣬������___________ ���á�
��2�����ø�װ�÷��������1-������������ƿA
�г�����1-�����������⣬��Ӧ�ȼ����������Լ�
___________�����ȵ�һ���¶ȣ��Թ�B���ռ������ǣ���д��ѧʽ��___________����ȴ��������ƿ�м����Լ�___________�ټ��ȵ�һ���¶ȣ��Թ�B���ռ�������___________����д��ѧʽ����
���ø�װ�ã����ü��ȣ�֤�����ԣ�����>̼��>���ӣ�������ƿA�м���___________ ���Թ�B��Ӧ����___________��
��1���ȼ���һ�������Ҵ���Ȼ���ҡ����ƿ������������һ������Ũ��������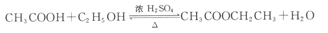 ����̼������Һ ������2����ʯ�� CH3CH2OH Ũ���� CH3COOH����3��̼���ƺʹ��� ������
����̼������Һ ������2����ʯ�� CH3CH2OH Ũ���� CH3COOH����3��̼���ƺʹ��� ������
���������������1����ȡ�����������Լ���������Ҵ�������Ҫ����Ũ����������������Լ�ʱҪ������Ũ���ᣬ���һ�Ҫ���Ͻ��裬��ΪŨ��������ˮʱҪ�ų��������ȣ�Ҫʹ������ʱɢʧ���Թ�BҪ�ñ���̼������Һ����Ϊ̼������Һ���Ժ����ᷴӦ�������ܽ��Ҵ������������ڱ���̼������Һ�е��ܽ�ȼ�С�����������ܴﵽ��������������Ŀ�ģ�����2����Ϊ�����1-�����ķе����С�����ܲ���ֱ������İ취��������������ƣ��Ȱ�����ת�������ӻ���������ƣ�����ʱ������ֻ���Ҵ����ټ����ᣬ�������ת�������ᣬ����ʱ�����ľ��������ˣ���3������ǿ���������ԭ����Ҫ֤���������̼�ᣬ��Ҫ�����Ƴ�̼�ᣬ�ʿ���ͨ�������̼�ᷴӦ����̼�ᣬ̼��ȶ����ֽ�ɶ�����̼��ˮ֤����Ҫ֤��̼����ڱ����轫������̼ͨ�뱽������Һ�У�̼���Ƴ�������֤����
���㣺



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ҩ�ﰢĪ������ɱ�������ϸ����ֳ�����ĺϳ�·�����£�
A��ʹ���Ȼ�����Һ��ɫ��
���������գ�
��1��д��A�Ľṹ��ʽ��_____________��CH3I������___________________��
��2����Ӧ�ܵĻ�ѧ����ʽ____________________________________��
H��һ��ͬ���칹���һ�ȴ���ĽṹΪ ����������NaOHˮ��Һ�м��ȷ�Ӧʱ�Ļ�ѧ����ʽΪ������������������������
����������NaOHˮ��Һ�м��ȷ�Ӧʱ�Ļ�ѧ����ʽΪ������������������������
��3����д����ͬʱ������������H���е�ͬ���칹������������������������
a�������Ȼ�����Һ������ɫ��Ӧ������b������̼�����Ʒ�Ӧ������ɫ����
c��ȡ0��1mol�л�����������Na��Ӧ�ܲ���3��36L������£�����
d�������ϵ�һ�ȴ���ֻ�����֣�����������ԭ��������3
e�������к��м�
��4��������CH3CH��CH2Ϊԭ���Ʊ� �ĺϳ�·������ͼ�����Լ����ã���
�ĺϳ�·������ͼ�����Լ����ã���
�ϳ�·������ͼ�����£�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��10�֣�д�����з�Ӧ�Ļ�ѧ����ʽ����ָ����Ӧ���ͣ�
��1��ʵ��������ϩ �� ��
��2���üױ��Ʊ�TNT �� ��
��3�����Ӻ�Ũ��ˮ�ķ�Ӧ �� ��
��4��1��2-�����������������������Ƶ��Ҵ���Һ���ȣ� ( )
��5����ȩ��������Һ�ķ�Ӧ �� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��5�֣���֪±������R-X���ڼ��������¿�ˮ��õ�����R-OH�����磺CH3CH2-X+H2O  CH3CH2-OH+HR����������ת����ϵ:
CH3CH2-OH+HR����������ת����ϵ: 
�ش��������⣺
��1����Ӧ1���Լ�������Ϊ __________��X�Ľṹ��ʽΪ______��Y�Ľṹ��ʽΪ______��
��2��д����Ӧ3�ķ���ʽ___________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��8�֣�±���������������о��й㷺��Ӧ�ã��ش��������⣺
��1����±��������Ϊ�ܼ������з��ӽṹΪ�����������__________����ҵ�Ϸ�����Щ��±������ķ�����_______________________��
��2�������������飨CF3CHClBr����һ����������д��������ͬ���칹��Ľṹ��ʽ__________(�����������칹)��
��3��������ϩ�������г��������ϡ���ҵ����������ϩ��һ�ֹ���·�����£�
��Ӧ�ٵĻ�ѧ����ʽ��_____________����Ӧ����Ϊ_______����Ӧ�ڵķ�Ӧ����Ϊ___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��Ҫ������������⣺
��ij�л���ļ���ʽ�� ����д������NaOH��Һ��Ӧ�Ļ�ѧ����ʽ ��
����д������NaOH��Һ��Ӧ�Ļ�ѧ����ʽ ��
��DDT����ϳɵĵ�һ���л���ũҩ������ӽṹ���ģ����ͼ��ʾ������������ʵĺ˴Ź���1H��ͼ���� �����շ塣
��F��G�� �� ���ճ������г��õ����ֺϳɸ߷��Ӳ��ϣ�����ij�������з�Ӧ�õ���
�� ���ճ������г��õ����ֺϳɸ߷��Ӳ��ϣ�����ij�������з�Ӧ�õ���
��ش��������⣺
��F�Ľṹ��ʽΪ ��
��C�����������ŵ�����Ϊ ���� �ǣ����ԲⶨD�����������š�
��A��B�Ļ�ѧ����ʽΪ ��
����֪2RCH(OH)COOH 2H2O +
2H2O + 
��ο���Ŀ�еĺϳ�;�������ϳ� ����ʼԭ�ϵ�ij���Ľṹ��ʽΪ �������� ����Ӧ�����Ժϳɲ��
����ʼԭ�ϵ�ij���Ľṹ��ʽΪ �������� ����Ӧ�����Ժϳɲ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼΪӲ֬��������ڼ���������ˮ���װ�á�����������Ӧʱ�IJ������£�
(1)��Բ����ƿ��װ��7��8 gӲ֬���������Ȼ�����2��3 g�������ơ�5 mLˮ��10 mL�ƾ�������ƾ�������Ϊ ��
(2)����ʯ��������Ӧ��������Լ10 min��������Ӧ������ɣ����õĻ����Ϊ (�����Һ����������Һ��������Һ�����塱)��
(3)�����û�����м��� (�ѧʽ)������һ��ʱ�䣬��Һ��Ϊ�������㣬������ �㣬���������Ϊ ��
(4)ͼ�г��������ܵ�����Ϊ ��
(5)д���÷�Ӧ�Ļ�ѧ����ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ʽΪC3H8O��Һ̬�л���A 1 mol���������Ľ��������ã���������11.2 L H2����״��������A�����бغ���һ��____________��������ŵ����ƣ������ù�����λ��̼����һ�ˣ���A�Ľṹ��ʽΪ____________��A��Ũ���Ṳ��170�����ϣ�������������ˮ�ķ�Ӧ���÷�Ӧ����ʽΪ__________________��A��ͭ������ʱ�����������ȣ���������C��д����������Ӧ�Ļ�ѧ����ʽ______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
������ͼת����ϵ��������˵������ȷ����
| A��N���� |
| B������ʽΪC4H8O2���л����ͬ���칹�干��5�� |
| C�����̢��������һ��ʱ��������ǼӴ�Ƭ��Ӧ��ֹͣ��Ӧ������ȴ�� |
| D�����ñ��͵�̼��������Һ���������������л��е����� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com