
 ����ͼ��ʾ��ʵ��װ���У�EΪһ���õ��ۡ��⻯�غͷ�̪�����Һ��ʪ����ֽ��C��DΪ������ֽ���˵IJ��У�X��Y�ֱ�Ϊֱ����Դ����������A��B�г���KOH��Һ������ʢ��KOH��Һ��ˮ���У��ٷֱ���벬�缫���жϵ�Դ����S1���պϿ���S2��ֱͨ����һ��ʱ�����ش��������⣺
����ͼ��ʾ��ʵ��װ���У�EΪһ���õ��ۡ��⻯�غͷ�̪�����Һ��ʪ����ֽ��C��DΪ������ֽ���˵IJ��У�X��Y�ֱ�Ϊֱ����Դ����������A��B�г���KOH��Һ������ʢ��KOH��Һ��ˮ���У��ٷֱ���벬�缫���жϵ�Դ����S1���պϿ���S2��ֱͨ����һ��ʱ�����ش��������⣺

 ͬ����ϰǿ����չϵ�д�
ͬ����ϰǿ����չϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ����ͼ��ʾ��ʵ��װ���У�A��ʢ��Ʒ����Һ��B ��ʢ��NaOH��Һ��
����ͼ��ʾ��ʵ��װ���У�A��ʢ��Ʒ����Һ��B ��ʢ��NaOH��Һ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
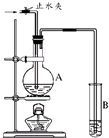 ����ͼ��ʾ��ʵ��װ���У�A��ʢ��Ʒ����Һ��B ��ʢ��NaOH��Һ��
����ͼ��ʾ��ʵ��װ���У�A��ʢ��Ʒ����Һ��B ��ʢ��NaOH��Һ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��6�֣�����ͼ��ʾ��ʵ��װ���У���A��B��C�ֱ�Ϊ���²�ͬ���ʱ���ش��й����⡣

��1����AΪ���ᡢBΪ���ǣ���ĩ����CΪˮ����ʱ��С�Թ��е������� �����з��������ӷ�Ӧ����ʽ�� ��
��2����AΪŨ��ˮ��BΪ��ʯ�ҡ�CΪAlCl3��Һ��С�Թ��г��ֵ������� ����Ӧ�����ӷ���ʽΪ ��
��3����AΪŨ���ᡢBΪMg��Al��Fe��Cu�е�һ������ɫƬ״������CΪƷ����Һ��С�Թ�����Һ��ɫ��ȥ����A��B��Ӧ�Ļ�ѧ����ʽ�� ��
��4��װ��D�������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�츣��ʡ�����а��ظ߶���ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ������
��1����֪��C3H8(g)+5O2(g) ===3CO2(g)+4H2O(1) ��H=��2220.0kJ/mol
H2O(1) ===H2O(g)����H=+44.0kJ/mol
д������ȼ������CO2����̬ˮ���Ȼ�ѧ����ʽ ��
��2������ͼ��ʾ��ʵ��װ���У�EΪһ���õ��۵⻯����Һ��ʪ����ֽ��C��DΪ������ֽ���˵IJ��У�X��Y�ֱ�Ϊֱ����Դ����������A��B�г���AgNO3��Һ������ʢ��AgNO3��Һ��ˮ���У��ٷֱ���벬�缫���жϵ�Դ����S1���պϿ���S2��ֱͨ����һ��ʱ���B�缫�������أ�A��������ɫ���塣��ش��������⣺
�� д��D�缫��Ӧʽ���������������� ��
��д��ˮ���е��AgNO3��Һ�ܷ�Ӧ�����ӷ���ʽ�� ����������2.16 g���������Ϸų��������ڱ�״���µ������____ _L��

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com