
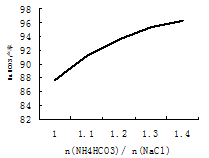


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

 ��1���ϳɰ���Ӧ��ӦN2��g��+3H2��g��
��1���ϳɰ���Ӧ��ӦN2��g��+3H2��g��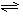 2NH3��g�������ں��¡���ѹ��������ƽ����ϵ��ͨ�������ƽ�� �ƶ�����������ҡ���������ʹ�ô��� ��Ӧ�Ħ�H���������С�����ı䡱����
2NH3��g�������ں��¡���ѹ��������ƽ����ϵ��ͨ�������ƽ�� �ƶ�����������ҡ���������ʹ�ô��� ��Ӧ�Ħ�H���������С�����ı䡱���� ��2����֪��N2H4��g��+O2��g��=N2��g��+2H2O��g������H=��543kJ��mol��1
��2����֪��N2H4��g��+O2��g��=N2��g��+2H2O��g������H=��543kJ��mol��1
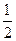 H2��g��+
H2��g��+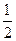 F2��g��=HF��g������H=��269kJ��mol��1
F2��g��=HF��g������H=��269kJ��mol��1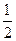 O2��g��=H2O��g������H=��242kJ��mol��1
O2��g��=H2O��g������H=��242kJ��mol��1 ��Ӧ N2H4��g��+2F2��g��=N2��g��+4HF��g����
��Ӧ N2H4��g��+2F2��g��=N2��g��+4HF��g����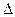 H="_____________" kJ��mol-1��
H="_____________" kJ��mol-1�� ��3����֪25��ʱKsp[Mg��OH��2]=1.8��10-11,KsP[Cu��OH��2]=2.2��10-20����25���£���Ũ�Ⱦ�Ϊ0.1 mol��L-1��MgCL2��CuCl2�����Һ����μ��백ˮ��������__________�������ѧʽ�������ɸó��������ӷ���ʽΪ______��
��3����֪25��ʱKsp[Mg��OH��2]=1.8��10-11,KsP[Cu��OH��2]=2.2��10-20����25���£���Ũ�Ⱦ�Ϊ0.1 mol��L-1��MgCL2��CuCl2�����Һ����μ��백ˮ��������__________�������ѧʽ�������ɸó��������ӷ���ʽΪ______�� ��4����25���£���a mol��L-1�İ�ˮ��0.01 mol��L-1������������ϣ���Ӧƽ��ʱ��Һ��c��NH4+��=c��Cl-��������Һ��_____________�ԣ���ᡱ������С������ú�a�Ĵ���ʽ��ʾ��ʱ��Һ��NH3��H2O�����ʵ���Ũ��__________ mol��L-1����˵������Һ���������ֱ����ӣ�
��4����25���£���a mol��L-1�İ�ˮ��0.01 mol��L-1������������ϣ���Ӧƽ��ʱ��Һ��c��NH4+��=c��Cl-��������Һ��_____________�ԣ���ᡱ������С������ú�a�Ĵ���ʽ��ʾ��ʱ��Һ��NH3��H2O�����ʵ���Ũ��__________ mol��L-1����˵������Һ���������ֱ����ӣ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
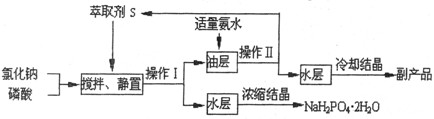
 NaH2PO4��HCl
NaH2PO4��HCl  HCl��S ��H��0
HCl��S ��H��0 H3PO4��S ��H��0
H3PO4��S ��H��0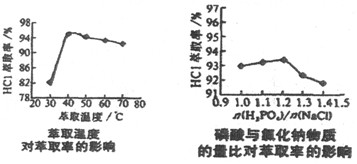
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 3NH3(g) ��H����92.2kJ/mol���ϳɰ���ҵ��ԭ����N2�ɴӿ����з���õ���H2����̼�ڸ�������ˮ������Ӧ�Ƶá��ҹ��ϳɰ���ҵĿǰ����������Ϊ������������ý���¶ȣ�400��500�棬ѹǿ��30��50MPa���ش��������⣺
3NH3(g) ��H����92.2kJ/mol���ϳɰ���ҵ��ԭ����N2�ɴӿ����з���õ���H2����̼�ڸ�������ˮ������Ӧ�Ƶá��ҹ��ϳɰ���ҵĿǰ����������Ϊ������������ý���¶ȣ�400��500�棬ѹǿ��30��50MPa���ش��������⣺ H2(g) + CO(g) ��H =" +131.3" kJ ����S =" +133.7" J/K
H2(g) + CO(g) ��H =" +131.3" kJ ����S =" +133.7" J/K 2NH3(g) ��K=0.5��
2NH3(g) ��K=0.5�� N2 (g)+ 3H2(g)��K= ������ֵ����
N2 (g)+ 3H2(g)��K= ������ֵ����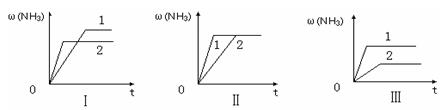
| A��ͼ������Dz�ͬѹǿ�Է�Ӧ��Ӱ�죬��P2��P1 |
| B��ͼ������Dz�ͬѹǿ�Է�Ӧ��Ӱ�죬��P1��P2 |
| C��ͼ������Dz�ͬ�¶ȶԷ�Ӧ��Ӱ�죬��T1��T2 |
| D��ͼ�������ͬ��ͬѹ�£��������ܣ�1��2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
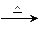 CO2��2H2O��4Cu����������ȫ��Ӧ��Ӳ�ʲ����ܵ���������4.8g������Ӧ�����������ͨ�������ij���ʯ��ˮ��������գ����ɳ���8.5g��
CO2��2H2O��4Cu����������ȫ��Ӧ��Ӳ�ʲ����ܵ���������4.8g������Ӧ�����������ͨ�������ij���ʯ��ˮ��������գ����ɳ���8.5g���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

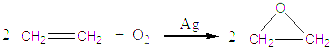
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
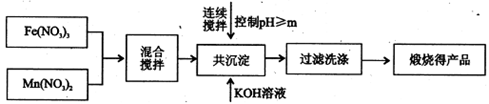
| | ��ʼ���� | ��ȫ���� |
| Fe3+ | 2.7 | 4.2 |
| Mn2+ | 8.3 | 10.4 |
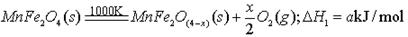
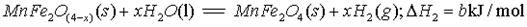
 (����ĸ���)��
(����ĸ���)�� B�����Ͽ�ѭ��ʹ��
B�����Ͽ�ѭ��ʹ��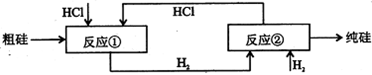
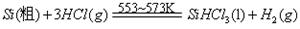
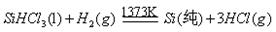
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����ҵ��ұ��������Ӳ�ʲ������н��е� |
| B����ҵ��ˮ�����������ˮ���תҤ�н��е� |
| C����ҵ���Ʊ�������Ҫ�õ��Ƚ����� |
| D����ҵ�ϻ�þ�ͭҪ�ڵ��װ���н��� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com