
 O2£Øg£©ØTCu2O£Øs£©”÷H=-169kJ?mol-1£¬
O2£Øg£©ØTCu2O£Øs£©”÷H=-169kJ?mol-1£¬ O2£Øg£©ØTCO£Øg£©”÷H=-110.5kJ?mol-1£¬
O2£Øg£©ØTCO£Øg£©”÷H=-110.5kJ?mol-1£¬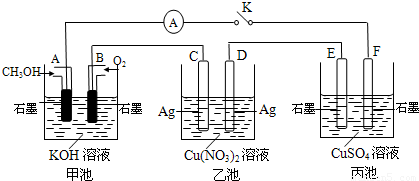
 O2£Øg£©ØTCu2O£Øs£©”÷H=-169kJ?mol-1£¬
O2£Øg£©ØTCu2O£Øs£©”÷H=-169kJ?mol-1£¬ O2£Øg£©ØTCO£Øg£©”÷H=-110.5kJ?mol-1£¬
O2£Øg£©ØTCO£Øg£©”÷H=-110.5kJ?mol-1£¬ ×¢Ł£¬·“Ó¦µÄģŹ±äŹĒ-110.5kJ?mol-1-£Ø-314kJ?mol-1£©-
×¢Ł£¬·“Ó¦µÄģŹ±äŹĒ-110.5kJ?mol-1-£Ø-314kJ?mol-1£©- ×£Ø-169kJ?mol-1£©=34.5kJ?mol-1£¬
×£Ø-169kJ?mol-1£©=34.5kJ?mol-1£¬ 2H2SO4+2Cu+O2”ü£¬¹Ź“š°øĪŖ£ŗŅõ¼«£»2CuSO4+2H2O
2H2SO4+2Cu+O2”ü£¬¹Ź“š°øĪŖ£ŗŅõ¼«£»2CuSO4+2H2O 2H2SO4+2Cu+O2”ü£»
2H2SO4+2Cu+O2”ü£»

 ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø
ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| 1 |
| 2 |
| 1 |
| 2 |
| ||
| ||
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| ||
| 1 |
| 2 |
| 1 |
| 2 |
| 1 |
| 2 |
| ||
| ¹āÕÕ |
| Cu2O |
| ŠņŗÅ | t/min | 0 | 10 | 20 | 30 | 40 | 50 |
| C/mol-1 | |||||||
| ĪĀ¶Č/”ę | |||||||
| ¢Ł | T1 | 0.500 | 0.492 | 0.486 | 0.482 | 0.480 | 0.480 |
| ¢Ś | T1 | 0.500 | 0.488 | 0.484 | 0.480 | 0.480 | 0.480 |
| ¢Ū | T2 | 0.500 | 0.450 | 0.450 | 0.450 | 0.450 | 0.450 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012-2013Ń§ÄźÉ½¶«Ą³ĪߏŠµŚŅ»ÖŠŃ§ø߶žÉĻŃ§ĘŚĘŚÄ©æ¼ŹŌ»ÆѧŹŌ¾ķ£Ø“ų½āĪö£© ĢāŠĶ£ŗĢīæÕĢā
£Ø1£© ÄÉĆ×¼¶Cu2OÓÉÓŚ¾ßÓŠÓÅĮ¼µÄ“߻ƊŌÄܶųŹÜµ½¹Ų×¢”£ŅŃÖŖ£ŗ
2Cu(s)+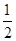 O2(g) ="==" Cu2O(s) ¦¤H=£169kJ”¤mol-1£¬
O2(g) ="==" Cu2O(s) ¦¤H=£169kJ”¤mol-1£¬
C(s)+ 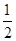 O2(g) ="==" CO(g) ¦¤H=£110.5kJ”¤mol-1£¬
O2(g) ="==" CO(g) ¦¤H=£110.5kJ”¤mol-1£¬
2Cu(s)+ O2(g)===2 CuO(s) ¦¤H=£314kJ”¤mol-1
Ōņ¹¤ŅµÉĻÓĆĢæ·ŪŌŚøßĪĀĢõ¼žĻĀ»¹ŌCuOÖĘČ”Cu2OŗĶCOµÄČČ»Æѧ·½³ĢŹ½ĪŖ
ӣ
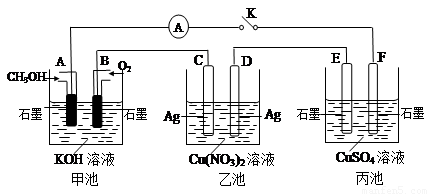
£Ø2£©Ä³ŠĖȤŠ”×éµÄĶ¬Ń§ÓĆĻĀĶ¼ĖłŹ¾×°ÖĆŃŠ¾æÓŠ¹Ųµē»ÆѧµÄĪŹĢā(¼×”¢ŅŅ”¢±ūČż³ŲÖŠČÜÖŹ×ćĮæ)£¬µ±±ÕŗĻøĆ×°ÖƵĵē¼üKŹ±£¬¹Ū²ģµ½µēĮ÷¼ĘµÄÖøÕė·¢ÉśĮĖĘ«×Ŗ”£
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©¼×³ŲĪŖ (Ģī”°Ōµē³Ų”±”¢”°µē½ā³Ų”±»ņ ”°µē¶Ę³Ų”±)£¬Aµē¼«µÄµē¼«·“Ó¦Ź½ĪŖ ”£
£Ø2£©±ū³ŲÖŠFµē¼«ĪŖ (Ģī”°Õż¼«”±”¢”°øŗ¼«”±”¢”°Ņõ¼«”±»ņ”°Ńō¼«”±)£¬øĆ³ŲµÄ×Ü·“Ó¦·½³ĢŹ½ĪŖ ”£
£Ø3£©µ±³ŲÖŠC¼«ÖŹĮæ¼õĒį10.8 gŹ±£¬¼×³ŲÖŠBµē¼«ĄķĀŪÉĻĻūŗÄO2µÄĢå»żĪŖ mL(±ź×¼×“æö)”£
£Ø4£©Ņ»¶ĪŹ±¼äŗ󣬶ĻæŖµē¼üK£¬ĻĀĮŠĪļÖŹÄÜŹ¹ŅŅ³Ų»Öø“µ½·“Ó¦Ē°ÅØ¶ČµÄŹĒ (ĢīŃ”Ļī×ÖÄø)”£
| A£®Cu | B£®CuO | C£®Cu(OH)2 | D£®Cu2(OH)2CO3 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2014½ģɽ¶«Ą³ĪߏŠø߶žÉĻŃ§ĘŚĘŚÄ©æ¼ŹŌ»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
£Ø1£© ÄÉĆ×¼¶Cu2OÓÉÓŚ¾ßÓŠÓÅĮ¼µÄ“߻ƊŌÄܶųŹÜµ½¹Ų×¢”£ŅŃÖŖ£ŗ
2Cu(s)+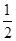 O2(g) ="==" Cu2O(s)
¦¤H=£169kJ”¤mol-1£¬
O2(g) ="==" Cu2O(s)
¦¤H=£169kJ”¤mol-1£¬
C(s)+ 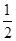 O2(g) ="==" CO(g)
¦¤H=£110.5kJ”¤mol-1£¬
O2(g) ="==" CO(g)
¦¤H=£110.5kJ”¤mol-1£¬
2Cu(s)+ O2(g)===2 CuO(s) ¦¤H=£314kJ”¤mol-1
Ōņ¹¤ŅµÉĻÓĆĢæ·ŪŌŚøßĪĀĢõ¼žĻĀ»¹ŌCuOÖĘČ”Cu2OŗĶCOµÄČČ»Æѧ·½³ĢŹ½ĪŖ
ӣ
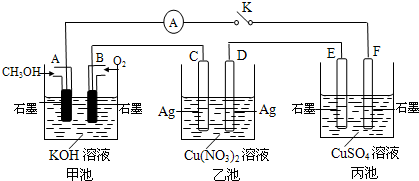
£Ø2£©Ä³ŠĖȤŠ”×éµÄĶ¬Ń§ÓĆĻĀĶ¼ĖłŹ¾×°ÖĆŃŠ¾æÓŠ¹Ųµē»ÆѧµÄĪŹĢā(¼×”¢ŅŅ”¢±ūČż³ŲÖŠČÜÖŹ×ćĮæ)£¬µ±±ÕŗĻøĆ×°ÖƵĵē¼üKŹ±£¬¹Ū²ģµ½µēĮ÷¼ĘµÄÖøÕė·¢ÉśĮĖĘ«×Ŗ”£
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©¼×³ŲĪŖ (Ģī”°Ōµē³Ų”±”¢”°µē½ā³Ų”±»ņ ”°µē¶Ę³Ų”±)£¬Aµē¼«µÄµē¼«·“Ó¦Ź½ĪŖ ”£
£Ø2£©±ū³ŲÖŠFµē¼«ĪŖ (Ģī”°Õż¼«”±”¢”°øŗ¼«”±”¢”°Ņõ¼«”±»ņ”°Ńō¼«”±)£¬øĆ³ŲµÄ×Ü·“Ó¦·½³ĢŹ½ĪŖ ”£
£Ø3£©µ±³ŲÖŠC¼«ÖŹĮæ¼õĒį10.8 gŹ±£¬¼×³ŲÖŠBµē¼«ĄķĀŪÉĻĻūŗÄO2µÄĢå»żĪŖ mL(±ź×¼×“æö)”£
£Ø4£©Ņ»¶ĪŹ±¼äŗ󣬶ĻæŖµē¼üK£¬ĻĀĮŠĪļÖŹÄÜŹ¹ŅŅ³Ų»Öø“µ½·“Ó¦Ē°ÅØ¶ČµÄŹĒ (ĢīŃ”Ļī×ÖÄø)”£
A£®Cu B£®CuO C£®Cu(OH)2 D£®Cu2(OH)2CO3
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
£Ø1£© ÄÉĆ×¼¶Cu2OÓÉÓŚ¾ßÓŠÓÅĮ¼µÄ“߻ƊŌÄܶųŹÜµ½¹Ų×¢”£ŅŃÖŖ£ŗ
2Cu(s)+![]() O2(g) === Cu2O(s) ¦¤H=£169kJ”¤mol-1£¬
O2(g) === Cu2O(s) ¦¤H=£169kJ”¤mol-1£¬
C(s)+ ![]() O2(g) === CO(g) ¦¤H=£110.5kJ”¤mol-1£¬
O2(g) === CO(g) ¦¤H=£110.5kJ”¤mol-1£¬
2Cu(s)+ O2(g)=== CuO(s) ¦¤H=£314kJ”¤mol-1
Ōņ¹¤ŅµÉĻÓĆĢæ·ŪŌŚøßĪĀĢõ¼žĻĀ»¹ŌCuOÖĘČ”Cu2OŗĶCOµÄČČ»Æѧ·½³ĢŹ½ĪŖ
ӣ
£Ø2£©Ä³ŠĖȤŠ”×éµÄĶ¬Ń§ÓĆĻĀĶ¼ĖłŹ¾×°ÖĆŃŠ¾æÓŠ¹Ųµē»ÆѧµÄĪŹĢā(¼×”¢ŅŅ”¢±ūČż³ŲÖŠČÜÖŹ×ćĮæ)£¬µ±±ÕŗĻøĆ×°ÖƵĵē¼üKŹ±£¬¹Ū²ģµ½µēĮ÷¼ĘµÄÖøÕė·¢ÉśĮĖĘ«×Ŗ”£
 |
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©¼×³ŲĪŖ (Ģī”°Ōµē³Ų”±”¢”°µē½ā³Ų”±»ņ ”°µē¶Ę³Ų”±)£¬Aµē¼«µÄµē¼«·“Ó¦Ź½ĪŖ ”£
£Ø2£©±ū³ŲÖŠFµē¼«ĪŖ (Ģī”°Õż¼«”±”¢”°øŗ¼«”±”¢”°Ņõ¼«”±»ņ”°Ńō¼«”±)£¬øĆ³ŲµÄ×Ü·“Ó¦·½³ĢŹ½ĪŖ
ӣ
£Ø3£©µ±³ŲÖŠC¼«ÖŹĮæ¼õĒį10.8 gŹ±£¬¼×³ŲÖŠBµē¼«ĄķĀŪÉĻĻūŗÄO2µÄĢå»żĪŖ mL(±ź×¼×“æö)”£
£Ø4£©Ņ»¶ĪŹ±¼äŗ󣬶ĻæŖµē¼üK£¬ĻĀĮŠĪļÖŹÄÜŹ¹ŅŅ³Ų»Öø“µ½·“Ó¦Ē°ÅØ¶ČµÄŹĒ (ĢīŃ”Ļī×ÖÄø)”£
A£®Cu B£®CuO C£®Cu(OH)2 D£®Cu2(OH)2CO3
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com