
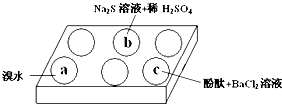
 ij��ѧС�����Na2SO3 ������ʵ��̽����
ij��ѧС�����Na2SO3 ������ʵ��̽����| ��� | ʵ������ |
| a | ��ˮ��ɫ |
| b | ��������ɫ���� |
| c | �����̪��Һ��죬�ټ���BaCl2��Һ����������Һ�ɫ��ȥ |
| n��SO32-����n��HSO3-�� | 91��9 | 1��1 | 9��91 |
| pH | 8.2 | 7.2 | 6.2 |


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�����ʡ����һ�и߶���ѧ����ĩ���Ի�ѧ�Ծ����������� ���ͣ�ʵ����
(14��)ij�о���ѧϰС�齫һ��Ũ�ȵ�Na2 CO3 ��Һ����CuSO4 ��Һ�еõ���ɫ��������ͬѧ��Ϊ���߷�Ӧֻ����һ�ֳ���CuCO3 ����ͬѧ��Ϊ������ٽ�ˮ�⣬ֻ����һ�ֳ���Cu(OH)2 ����ͬѧ��Ϊ����CuCO3 ��Cu(OH)2���ֳ������۲�������֪��CuCO3 ��Cu(OH)2 �������ᾧˮ��
��.������ͬѧ�����⣬Na2 CO3 ��Һ��CuSO4 ��Һ��Ӧ�Ļ�ѧ����ʽΪ ��
��̽��������ɷ�ǰ���뽫��������Һ�з��벢��������������ٹ��ˡ���ϴ�ӡ��۸��
��.������ͼ��ʾװ�ã�ѡ���Ҫ���Լ�������̽��������ijɷ֡�
(1)��װ������˳��Ϊ ��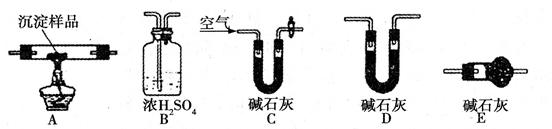
(2)װ��C����װ�Լ��������� ��
(3)��֤������������CuCO3 ��ʵ�������� ��
��.��CuCO3 ��Cu(OH)2���߶��У���ͨ��������ʾװ�ý��ж����������ⶨ����ɡ�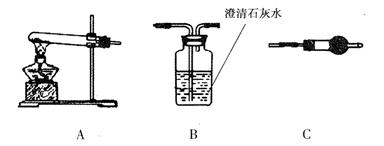
(1)װ��C�м�ʯ�ҵ������� ��ʵ�鿪ʼ��ʵ�����ʱ��Ҫͨ������Ŀ�������ʵ�����ʱͨ������������� ��
(2)��������Ʒ������Ϊm g��װ��B������������n g���������CuCO3����������Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�����ʡ�߶���ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
(14��)ij�о���ѧϰС�齫һ��Ũ�ȵ�Na2 CO3 ��Һ����CuSO4 ��Һ�еõ���ɫ��������ͬѧ��Ϊ���߷�Ӧֻ����һ�ֳ���CuCO3 ����ͬѧ��Ϊ������ٽ�ˮ�⣬ֻ����һ�ֳ���Cu(OH)2 ����ͬѧ��Ϊ����CuCO3 ��Cu(OH)2���ֳ������۲�������֪��CuCO3 ��Cu(OH)2 �������ᾧˮ��
��.������ͬѧ�����⣬Na2 CO3 ��Һ��CuSO4 ��Һ��Ӧ�Ļ�ѧ����ʽΪ ��
��̽��������ɷ�ǰ���뽫��������Һ�з��벢��������������ٹ��ˡ���ϴ�ӡ��۸��
��.������ͼ��ʾװ�ã�ѡ���Ҫ���Լ�������̽��������ijɷ֡�
(1)��װ������˳��Ϊ ��
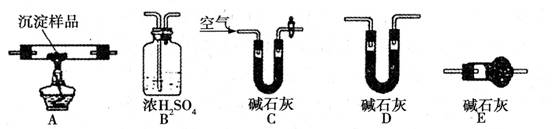
(2)װ��C����װ�Լ��������� ��
(3)��֤������������CuCO3 ��ʵ�������� ��
��.��CuCO3 ��Cu(OH)2���߶��У���ͨ��������ʾװ�ý��ж����������ⶨ����ɡ�

(1)װ��C�м�ʯ�ҵ������� ��ʵ�鿪ʼ��ʵ�����ʱ��Ҫͨ������Ŀ�������ʵ�����ʱͨ������������� ��
(2)��������Ʒ������Ϊm g��װ��B������������n g���������CuCO3����������Ϊ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com