


 �ο�ͨ�γ̱�˼ά����������ѵ��ϵ�д�
�ο�ͨ�γ̱�˼ά����������ѵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| Ũ���� |
| �� |
| Ũ���� |
| �� |


 ����1��
����1�� ����1��
����1���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| Ũ���� |
| �� |
| Ũ���� |
| �� |




�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| Ũ���� |
| ���� |
| Ũ���� |
| ���� |


 д������֮һ����
д������֮һ���� д������֮һ����
д������֮һ����| ʵ���� | C���ʵ���Ũ�ȣ�mol?L-1�� | NaOH���ʵ���Ũ�ȣ�mol?L-1�� | �����Һ��pH |
| m | 0.1 | 0.1 | pH=9 |
| n | 0.2 | 0.1 | pH��7 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�







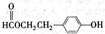
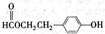
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
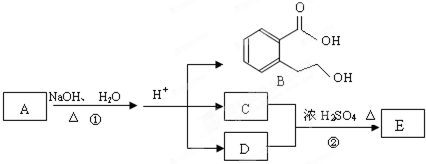
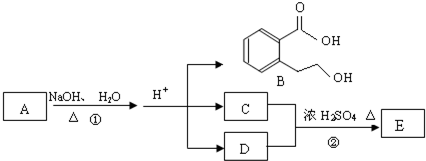
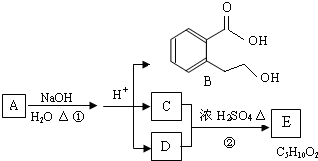
ij�л���A��ֻ��C��H��O����Ԫ�أ���һ����Ҫ�����������м��塣��֪��
��A�������ܶ�����ͬ״���������ܶȵ�83����������̼ԭ����������ԭ��������3����
��A���ڷ����廯����䱽����ֻ��һ��ȡ��������ȡ����̼������֧����
��A����NaHCO3��Һ���ã�������ɫ���ݣ�
��A��һ�������¿������ᷢ��������Ӧ��
��A��������ת����ϵ��
�Ը���������Ϣ�ش����⣺
��1��A�ķ���ʽ ��A�к��������ŵ����� ��
��2��D�Ľṹ��ʽΪ ��
��3��д��A��C��A��B�Ļ�ѧ��Ӧ����ʽ��ע����Ӧ����������ע����Ӧ���ͣ�
A��C�� _____________________________________��
��Ӧ���ͣ� ��
A��B�� ______________________________________��
��Ӧ���ͣ� ��
��4��������������������A��ͬ���칹�����Ŀ���� ����
�ٱ�����ֻ��������λȡ����
���������Ȼ�����Һ������ɫ��Ӧ
��һ�������¿���ˮ��������������
д����������һ��ͬ���칹��Ľṹ��ʽ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com