
| y |
| 4 |
| y |
| 4 |
| y |
| x |
| y |
| 4 |
| z |
| 2 |
| y |
| 4 |
| y |
| 4 |
| y |
| x |
| y |
| 4 |
| z |
| 2 |
| y |
| 4 |
| y |
| 4 |
| z |
| 4 |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
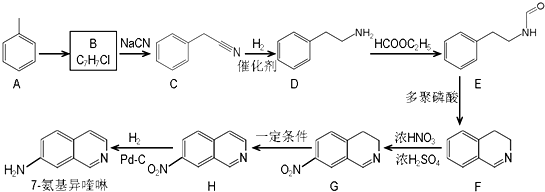
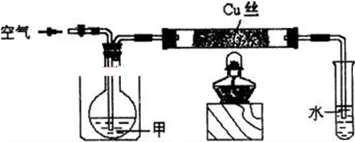
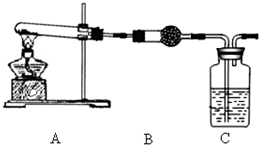
 ����ش��������⣺
����ش��������⣺ ��������ƺϳ�·�ߣ����Լ����ܼ���ѡ����
��������ƺϳ�·�ߣ����Լ����ܼ���ѡ����| O2 |
| ����/�� |
| �Ҵ� |
| Ũ����/�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��Fe3+��Fe2+�����ӹ������ۣ����� |
| B��Mg2+��Al3+�����ӹ�����ˮ������ |
| C��CO2��HCl����ͨ��̼���Ʊ�����Һ��ϴ�� |
| D������Na2CO3��NaHCO3�������������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���Ȼ��ƺ��Ȼ�þ��Һ�ֱ�����������Һ��϶��ܲ�����ɫ���� |
| B��Ũ�����ϡ���᳤�ڱ�¶�ڿ�����Ũ�Ƚ��� |
| C����ˮ�ͻ���̿ʹ��īˮ��ɫ |
| D��Ư�ۺ�ˮ�������ڱ�¶�ڿ����б��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
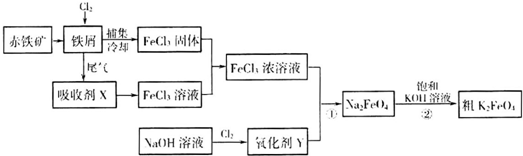
| �ŵ� |
| ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
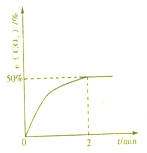 ������CO��SO2�̵�����Ⱦ��һ�ַ������ǽ����ڴ���������ת��Ϊ����S��g����������ӦΪ��2CO��g��+SO2��g��?S��g��+2CO2��g��
������CO��SO2�̵�����Ⱦ��һ�ַ������ǽ����ڴ���������ת��Ϊ����S��g����������ӦΪ��2CO��g��+SO2��g��?S��g��+2CO2��g��| 1 |
| 2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��4�� | B��5�� | C��6�� | D��7�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com